the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Temporal and vertical changes in snow microbial communities during the melting season below canopy in Northern Japan
Kino Kobayashi
Daiki Seto
Fuki Konishi
Kaito Wada
Suzunosuke Usuba
Nozomu Takeuchi
During snowmelt, diverse cold-tolerant microbes thrive within snowpacks. Snow conditions in forested areas change temporally with air temperature and budburst of trees. However, their effects on relevant biological communities are not well documented. Based on periodic sampling throughout the snowmelt season (March–May 2021), this study describes the temporal and vertical changes in biological communities, including snow algae, microinvertebrates, and snow fungi, within snowpacks in Northern Japan. The melting season was divided into three periods: when the daily minimum air temperature was below the freezing point (Period A), when it was above the freezing point and before the budburst of beech trees (Period B), and after the budburst over the snow surface (Period C). During Period A, two types of algae and one of fungus were ubiquitously observed in the snowpack. During Period B, the abundance of microbes increased in the surface layer and green algal blooms visibly emerged. Later in this period, nutrients (NO, NH, and PO) depleted, likely inhibiting algal growth and consequently restricting the microinvertebrate population. Surface layer nutrient concentrations increased again during Period C, thereby increasing the abundance of algae and microinvertebrates. This increase in nutrients was likely due to the rainwater and tree-derived litter deposited on the snowpack. Analyses of snow pits and cores revealed that the active layers of microbes were distinct between snow algae/fungi (surface layer) and microinvertebrates (subsurface layers), probably because of their preferable conditions. This study highlights potentially important patterns in the dynamic interactions between microbial communities and environmental changes within snowpacks, revealing how tree phenology and snowmelt conditions jointly shape the vertical distribution and seasonal succession of snow-ice microbes.
- Article
(6352 KB) - Full-text XML
-
Supplement
(2215 KB) - BibTeX
- EndNote
Despite the threat of its disappearance (Brown et al., 2017; Kawase et al., 2020, 2023), seasonal snow provides a habitat for various cold-tolerant microbes. The microbes, referred to as snow-ice microbes, include snow algae (Hoham and Duval, 2001; Hoham and Remias, 2020), microinvertebrates (e.g., tardigrades and rotifers; Hanzelová et al., 2018; Ono et al., 2021, 2022; Yakimovich et al., 2020), invertebrates (e.g., springtails and winter stoneflies; Negoro, 2009; Hao et al., 2020), fungi (Irwin et al., 2021; Matsuzaki et al., 2021; Nakanishi et al., 2023), and bacteria (Segawa et al., 2005; Amato et al., 2007). During the snowmelt season, snow algae often bloom in snowpacks and change the color of the snow surface to green, red, yellow, or orange (Tanabe et al., 2011; Remias et al., 2013; Procházková et al., 2019b, 2021; Hoham and Remias, 2020; Matsuzaki et al., 2022; Raymond et al., 2022). Active interspecies interactions such as microinvertebrate predation and fungal parasitism have been observed in colored snow (Ono et al., 2021; Nakanishi et al., 2023). Thus, seasonal snowpacks can be regarded as unique ecosystems (Domine, 2019).
In snowpack ecosystems, materials such as carbon, nitrogen, and phosphate are circulated through the metabolism of various microbes. These nutrients are deposited by snowfall, rain, airborne dusts, fire ash, and then transformed through freeze–thaw cycles, microbial processes, and hydrological movements within the snowpack (Jones, 1999). Photosynthetic microbes, such as snow algae and cyanobacteria, produce organic matter from inorganic carbon, nitrogen, and phosphorus sources. Especially in relation to nitrogen, studies from the Arctic have shown that the concentration of NO in snowpacks increased through nitrification, and subsequent denitrification or nitrification released NO into the atmosphere (Amoroso et al., 2010). In spring, when snow begins to melt, ammonification and assimilation of NH dominate the snowpack (Larose et al., 2013). Through predation and parasitic relationships reported previously (Ono et al., 2021; Nakanishi et al., 2023), the nitrogen and carbon contained in algal cells are transferred to other organisms. In forested areas, leachate from organic matter (e.g., balsam fir leaf) deposited by trees during the late snowmelt season contains nutrients for algal growth (Jones, 1991, 1999; Hoham et al., 2008). However, many uncertainties remain regarding when and where these biological activities begin within snowpacks and for how long they persist, and the factors influencing their distribution remain poorly understood.
Previous studies have only partially investigated the distribution of microbes in snowpacks and the factors that determine their distribution. For instance, snow algae bloom on nutrient-rich snowpack surfaces (Jones, 1991). They are not percolated into deeper layers by meltwater, but remain at the surface layer of the snowpacks even if surface melting occurs during the daytime (Grinde, 1983). Heterotrophic organisms such as microinvertebrates are also concentrated on the snow surface because they prefer to eat snow algae, which are abundant in the surface layer (Ono et al., 2021). The heterogeneous distribution of meltwater within snowpacks may influence the vertical distribution of microbes. For example, snow algae germinate at the soil surface or firns below snowpacks through the supply of meltwater and migrate upward (Jones, 1991; Hoham and Duval, 2001; Matsumoto et al., 2024; Rea and Dial, 2024). Snow algae are sometimes concentrated above the subsurface ice layers formed by refrozen meltwater within snowpacks (Hoham, 1975b). The vertical distribution of snow algae and microinvertebrates within snowpacks may vary depending on the intensity of solar radiation (Ono and Takeuchi, 2025).
The environmental conditions that influence snow-ice microbes change dynamically throughout the snowmelt season, thereby affecting their distribution and activity. During the transition from winter to spring, rising temperatures increase the water content of snowpacks, thereby promoting microbial mobility (Felip et al., 1995). Snow grains metamorphose into granular structures, and meltwater percolates through the snow layers, allowing microbes to migrate within the snowpacks via these meltwater (Hoham and Duval, 2001; Cruaud et al., 2020; Détain et al., 2025). Variations in snow depth and density further influence microbial habitats and determine their exposure to light and nutrient availability (Curl et al., 1972; Ono and Takeuchi, 2025). In forested areas, tree budburst can lead to a decrease in solar radiation. However, there is a lack of high-frequency, continuous observations linking these environmental changes to microbial dynamics within snowpacks during the season.
Diverse snow-ice microbes have been observed in snowpacks in the mountainous areas of Japan, where heavy snowfall occurs every winter due to Asian monsoons (Fukushima, 1963). Continuous sampling of surface snow during the snowmelt season has shown that the abundance of snow algae increases exponentially until snowpack disappearance (Onuma et al., 2016). Another study showed that algal abundance increases after the budburst of trees, with an increase in nutrient concentrations on the snowpack surface (Suzuki and Takeuchi, 2023). A recent study revealed that snow ice microbes actively inhabit the subsurface layers within snowpacks, referred to as the microbially active snow surface layer (MASS layer, 30 cm in depth, Ono and Takeuchi, 2025). In this study, temporal and vertical changes in microbial communities and chemical conditions of snowpacks in forested areas of Japan throughout the melting season were described to understand the environmental factors driving microbial activities within snowpacks.
2.1 Study site and sample collection
Sampling was conducted at Yumiharidaira Park (38°30′ N, 140°00′ E; 770 m a.s.l.) on Mt. Gassan, Yamagata Prefecture, Japan (Fig. 1). This site is suitable for investigating the relationship between tree phenology and snow-ice microbial communities as it hosts a diverse range of microbes (Tanabe et al., 2011; Ono et al., 2021, 2022; Suzuki and Takeuchi, 2023; Ono and Takeuchi, 2025) and retains snow after mid-May, when beech trees begin to sprout leaves (Suzuki and Takeuchi, 2023). Because of the monsoon, large amounts of snow accumulate during winter and snow persists until early summer (Kariya, 2002, 2005). The vegetation on Mt. Gassan changes from forest to alpine plants as the elevation increases. Broad-leaved and coniferous trees dominate below the forest line (1400–1500 m a.s.l.), followed by dwarf forests above 1500 m a.s.l. (Kariya, 2005). Meteorological conditions, including air temperature and solar radiation, were monitored at the snow surface within the basin during the spring season of 2021. The air temperature was recorded from 27 March to 19 May using a temperature/humidity data logger (TR-72nw; T&D Corporation, Japan). The data logger was suspended 50 cm above the snow surface with the help of a probe. It is recorded every hour from 27 March to 24 April, and every 10 min after 25 April. The intensity of the solar radiation was recorded every 10 s from 5 to 17 May using a pyranometer (ML-020VM; EKO, Japan) equipped with a data logger (LR5091; HIOKI, Japan). The hourly means of air temperature and solar radiation were obtained from the observations. The data logger was placed in areas with and without trees above the snow surface (referred to as tree-covered and -free areas, respectively) and held at a height of 5 cm above the snow surface with the help of a small pedestal. To describe temporal changes in the microbial community structure and chemical solutes within the snowpack, snow samples were collected during the snowmelt season of 2021. Snow samples were collected 11 times (on 7 and 27 March; 10, 24, and 28 April; and 4, 9, 12, 16, 18 and 20 May). Sampling was conducted at approximately 05:00 a.m. (Japan standard time) to eliminate the possibility of the sunlight-led disappearance of microbes from the snow surface (Hoham, 1975b; Kawecka, 1986; Ono and Takeuchi, 2025). First surface green algal blooms appeared on 28 April. The budburst of beech trees in the study area occurred between 12 and 15 May (Figs. 1, 2). Rainfall occurred on 25, 29 April and 2, 10, 17, and 19 May. Snow samples were collected from five layers across snow depths with a new pit for each sampling (Fig. 2): an area of 10 × 10 cm2 with depths of 0–3 cm (Layer I), 3–8 cm (Layer II), 8–13 cm (Layer III), 13–18 cm (Layer IV), and 18–23 cm (Layer V). These samples were collected at three different locations at each sampling time, except on 7 March, when they were collected at only one of the three locations. After the appearance of green algal blooms, samples were collected from one blooming snow surface (mainly green snow) and from two snow surfaces adjacent to the bloom. In total, 465 snow samples were collected using a small spatula and preserved in Whirl-Pak bags (B01065WA; Nasco, USA). To avoid potential interference with microbial distribution due to sampling, each sample was collected at a distance of at least 3 m from the others.

Figure 1Temporal changes at the study site during the study period. Temporal changes at the study site during the study period. The mean canopy height of beech forests in this region is 12.6 m (Shoji and Yoshimura, 2025). All the photos were taken in the morning (between 05:00 and 10:00).
To determine the vertical distribution of microbes at the bottom of the snowpack, snow cores were collected from the surface to the bottom of the snowpack using a hand auger manufactured by the Institute of Low Temperature Science, Hokkaido University, Japan (length: 1 m, diameter: 90 mm). Snow core collections were conducted at 05:00 a.m. on 25 April (core length: 180 cm), 4 May (133 cm), 7 May (113 cm), 10 May (94 cm), 13 May (80 cm), 16 May (56 cm), and 18 May (30 cm). The results of the snow core analyses collected on May 7th have been published (Ono and Takeuchi, 2025). The cores were cut horizontally every 10 cm using a snow saw and preserved in Whirl-Pak bags.
To evaluate the nutrient supply from rainwater in the snowpack, rainwater was collected twice at the study site. Samples were collected from both open and tree-covered areas on 3 May when beech leaves were partially open, and on 17 May when beech leaves were fully open. Samples were collected directly into a 50 mL polypropylene bottle attached to a stainless-steel rod.
All the samples were frozen and transported to a laboratory at Chiba University, Japan. The samples were stored in a freezer (−20 °C) until further processing. The samples slowly melted in a refrigerator (5 °C) prior to analysis for minimizing changes in microbial activity and chemical composition. After melting, the snow samples were separated as follows: 5 mL for chlorophyll a, 5 mL for chemical analysis after passing through a sieve (US mesh #40) to remove plant litter, 10 mL for the dry weight of total insoluble particles, and the remainder for microinvertebrates. Snow core samples, including snow algae and microinvertebrates, were used for microbial analysis. Samples for chemical analysis were separated into 6 mL plastic tubes after the meltwater was passed through a 0.45 µm ion-free disposable filter (13AI Chromatodisk; GL-science, Japan) to remove microbes and particulate dust.
2.2 Community structure analysis of microbes
To identify the temporal changes in the abundance of snow algae and fungi, measurements of chlorophyll a concentration and microscopic observations were conducted. Chlorophyll a is a common indicator of algal biomass (Holm-Hansen et al., 1965), and another method was described by Ono et al. (2021). To calculate the cell concentration of snow algae and fungi, 5–200 µL of each subsample was used. The samples were filtered through a filter holder equipped with a 0.45 µm PTFE membrane filter (JHWP01300; Merck Millipore, Germany) and a pump (Linicon LV-125; Nitto Kohki, Japan). The filter was then covered with a cover glass and MilliQ water on a glass slide. Cell counts were performed three times manually, and the mean cell number and sample volume used for filtration were used to calculate the cell concentration per water equivalent of the snow sample (cells L−1). For snow core samples, the vertical distributions are described as the proportions of abundance in each layer relative to all layers of the snow core. Particularly in snow algae, identification at the species level was not performed in this study because species identification based on the morphology of frozen samples is difficult. Therefore, the observed algae were classified into morphological types.
To analyze the abundance of microinvertebrates, the melted snow sample remaining after sample processing was transferred to a Petri dish. Tardigrades and rotifers were counted using a stereomicroscope (MZ125; Leica Microsystems, Germany). The population density of the microinvertebrates (individuals L−1) was calculated using the number of observed microinvertebrates and the water equivalent of the snow sample. Some individuals were mounted onto glass slides in a drop of Hoyer's medium (Degma, 2018) and observed under a phase-contrast microscope (BX51; Olympus, Japan) to identify the species, based on the description by Ono et al. (2021, 2022). The body size distribution of tardigrades in white snow on 24 April and alga-blooming snow was measured using the pictures taken by a digital camera (DP21; Olympus, Japan) and Image J 1.52 software. Body length was measured without leg IV length, and body width was measured between legs II and III (Ono et al., 2021).
2.3 Elution from tree-derived litter
Elution experiments were conducted to investigate the leaching of chemical solutes from plant litter into meltwater in the snowpack. Dried plant litter (0.05 g) from green algal-blooming snow samples from 20 May was placed in plastic Petri dishes. MilliQ water (50 mL) was added to each dish, and 5 mL was collected after 10 min, 24, 72, 120, 168, 240, and 336 h. The water samples were filtered using a 0.45 µm ion-free disposable filter with a 5 mL syringe and then preserved in 6 mL plastic tubes.
2.4 Analysis of chemical solutes and insoluble particles
To elucidate the relationship between snow-ice microbes and environmental conditions, the concentrations of chemical solutes and the abundance of insoluble particulates in the samples were analyzed. The concentrations (µEq kg−1) of major chemical solutes (cation: Na+, NH, K+, Ca2+, Mg2+; anion: Cl−, SO, NO, NO, PO) were quantified using an ion chromatography system (anion: AQUION, Thermo Fisher Scientific, USA; cation: ICS-1100, Thermo Fisher Scientific, USA). The NO levels were below the detection limit in all samples in this study. The dry weights of insoluble particulates in the samples were quantified by dividing them into three components (plant litter, organic matter, and inorganic matter). The plant litter in the samples was collected using a sieve and placed in a plastic Petri dish. They were then dried at 60 °C for 1–2 d until they reached a constant weight, and weighed. Ten milliliters of the remaining sample were transferred to ceramic crucibles. The crucibles were then dried at 60 °C for 1–2 d and subsequently subjected to combustion at 500 °C for 3 h in an electric furnace (SSTR-13R; ISUZU, Japan); only inorganic matter remained in the crucibles. The mass concentrations (g L−1) of organic and inorganic matter in the samples were calculated from their dry weights.
2.5 Statistical analysis
Welch's t-test was used to examine the differences in cell concentration, population density, chlorophyll a concentration, chemical solutes, and dry weights of insoluble particles between green and white snow. The test was applied to both the entire snow pit and surface layer (I). Welch's t-tests were used to compare the concentrations of chemical solutes in rainwater between tree-covered and -free areas before and after budburst. Differences in the body lengths of tardigrades between 24 April and 20 May were also assessed using the same test. Pearson's correlation coefficients were obtained for each microbe and environmental condition. Canonical Correspondence Analyses (CCA) were used to reveal the relationships between microbes and the chemical conditions within the snowpack. All statistical analyses were performed using the R version 4.3.3 (R Core Team, 2024).
3.1 Meteorological conditions at the study site during snowmelt
Snow depth, measured as the actual physical depth of the snowpack, was 320 cm on 6 March and 280 cm by 27 March (Fig. 3a). Snowfall occurred around 9 April, resulting in fresh snow cover of approximately 8 cm in depth (the snow depth was 223 cm at this time). Between 24 April and 4 May, the snow depth gradually decreased from 152 to 132 cm. The remaining snow depth was 6 cm on 20 May.
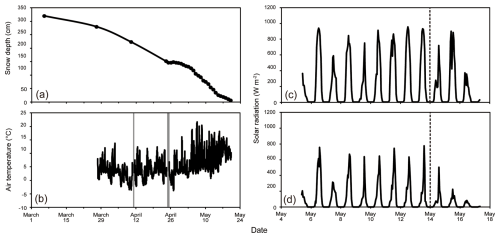
Figure 3Meteorological conditions recorded at the study site. (a) Snow depth and (b) air temperature in the tree-covered area during the study period (1 March to 24 May). Gray-shaded periods represent intervals of missing data caused by instrument maintenance. Hourly mean intensity of solar radiation on the snow surface in the (c) tree-free and (d) tree-covered areas from 4 to 16 May. The timing of beech tree budburst is indicated by the dashed lines in (c) and (d).
The air temperature was above 0 °C during daytime since the beginning of the study period (Fig. 3b). The minimum temperature was recorded in the morning (02:00–05:00), which was occasionally below the freezing point. After 27 April, the minimum temperatures were never below the freezing point and gradually increased until the end of May. The daily mean solar radiation on the snow surface in a tree-free area ranged from 100.2 to 305.5 W m−2, exhibiting small trends of increase or decrease during the measurement period (Fig. 3c). In contrast, that in a tree-covered area varied from 81.0 to 185.3 W m−2 (55 %–64 % of that of tree-free area) until 11 May and subsequently decreased to 22.5 W m−2 (22 % of that of tree-free area) on 16 May (Fig. 3d). This subsequent decrease matched the timing of beech tree budburst above the snow surface.
The study period was divided into three phases based on the forest conditions: Period A (7 March–27 April), in which the daily minimum temperature was below the freezing point; Period B (28 April–14 May), in which the daily minimum temperature was above the freezing point and the beech trees in the forest had not yet budded; and Period C (15–20 May), in which the daily minimum temperature was above the freezing point and the trees had sprouted.
3.2 Snow-ice microbes in the snowpack
Microscopic observations of snow samples revealed various microbes, as some overlap with Ono and Takeuchi (2025). They were morphologically identified using microscopy as snow algae Types A, B, C, and D; tardigrades (Hypsibius nivalis, Hypsibius sp.); rotifers (Philodinidae gen. sp.); and snow fungi (Chionaster (Chi.) nivalis). Snow algae types A, B, and C are the same as those described in Ono and Takeuchi (2025), and they represent various species of the genus Chloromonas. Type A is characterized by a spherical shape, Type B by an oval shape, and Type C by an oval shape with ribbed walls. In addition, Type D was observed in this study, which is barrel-shaped with projections at the corners. The length of this alga ranged from 14.7 to 21.3 µm (18.3 ± 1.4 µm), and the width from 10.3 to 15.0 µm (12.9 ± 1.1 µm). This corresponds to the zygote stage of Oocystis lacustris f. nivalis reported in previous studies (Fukushima, 1963; Matsuzaki et al., 2022).
3.3 Temporal changes in vertical patterns of abundance of snow-ice microbes
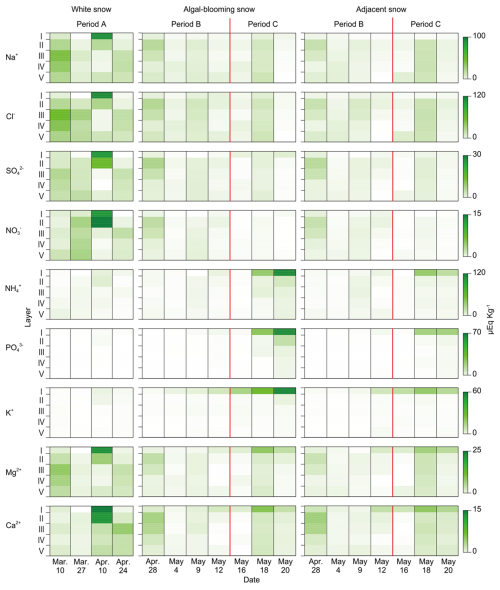
The chlorophyll a concentration in the upper layers above 13 cm generally increased throughout the study period (Fig. 4). Chlorophyll a was detected in the subsurface layers (III and V) for the first time (10 March, Period A). Subsequently, it increased mainly in Layer I during Period A. In the case of algal-blooming and adjacent snow, chlorophyll a increased in Layers I and III at the beginning of Period B. During Period C, the chlorophyll a content increased continuously in Layers I and II.
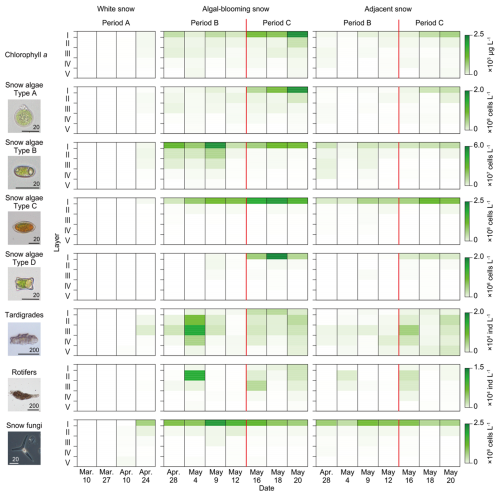
Figure 4Temporal and vertical changes in microbial abundance in the upper layers of the snowpack. Temporal and vertical changes in algal-blooming and adjacent snow surfaces were observed after the emergence of algal blooming. The red line indicates the time from which on the forest trees sprouted.
Temporal and vertical changes in snow algae abundance showed different trends for each morphotype. Type A showed a trend similar to that of chlorophyll a. Type B was first observed in Layers II and III in the middle of Period A (10 April). It then increased drastically in Layers I–III until the middle of Period B, but only in the algal-blooming snow. It decreased towards the end of Period B, but increased again in Period C. Types C and D were first observed at the end of Period A (24 April) in Layers I–II and I–III, respectively. They continuously increased in Layer I of the alga-blooming snow throughout Periods B and C. In the adjacent snow, Type B was distributed over the entire snowpack on 28 April, whereas it was concentrated on the snow surface in the alga-blooming snow. Types A, C, and D in the adjacent snow showed similar temporal and vertical distribution patterns to those observed in the alga-blooming snow.
The two microinvertebrates showed an appearance pattern distinct from that of the snow algae within the snowpack. They mainly appeared in the subsurface layers (II and III), and their populations varied significantly throughout the study period. Tardigrades and rotifers first appeared in the middle of Period A in Layer V (27 March) and layers III–V (24 April), respectively. Subsequently, their abundance increased in all layers during Period B (4 May) but decreased by the end of it. They increased again in all layers during Period C. In the adjacent snow, the vertical distributions of tardigrades and rotifers differed from those of the algal-blooming snow, with no increase in Layers I–IV observed in the alga-blooming snow on 4 May, whereas the vertical distribution was similar for the remainder of the period.
Snow fungi also showed a distinct pattern of appearance from those of algae or microinvertebrates; they appeared mostly in the surface layers and increased during Period B but decreased in Period C. Few individuals of Chi. nivalis appeared in all layers (I–V) in the middle of Period A (27 March). In both the algal-blooming and adjacent snow, their abundance in the surface layers (I and II) increased until the middle of Period B (9 May), and then decreased in Period C.
The abundance of microbes was two to five times higher in the algal-blooming snow than in the adjacent snow. The contrast was more significant in the surface layer (I), and their abundance was 2–6 times higher than that in the adjacent snow. However, this was not observed for Type D algae or tardigrades (Table S1 in the Supplement).
3.4 Temporal changes in snow-ice microbes
Analysis of the snow core samples revealed that the snow-ice microbes (snow algae, tardigrades, and rotifers) were concentrated in the layers from the surface to the depth of 30 cm during the study period (Fig. S1). Chlorophyll a concentrations were significantly higher in the surface layers above the depth of 30 cm than in the lower layers from late April to early May (73 %–98 % of the total content in the snow core). After mid-May, they were significantly higher in the layers above the depth of 10 cm (68 %–82 %). Tardigrades were mostly concentrated in the surface layers above the depth of 20 cm during the study period (71 %–95 %). Rotifers were also concentrated in the surface layers above the depth of 20 cm during these periods (74 %–100 %); however, they were exceptionally found at the 120–130 cm depth on 25 April and 60–70-cm depth on 13 May.
3.5 Temporal changes in body size distribution and species composition of tardigrades within the algal-blooming snowpack
The mean body length of the tardigrades increased over time and their size distributions differed between the snow surface and subsurface layers (Fig. 5). Tardigrade body lengths in the subsurface layers ranged from 210 to 423 µm on 24 April, and significantly increased to 242–444 µm by the final sampling on 20 May (t= 6.28, p<0.01). A similar trend was observed on the snow surface; however, only smaller individuals (< 200 µm) were observed on the snow surface.
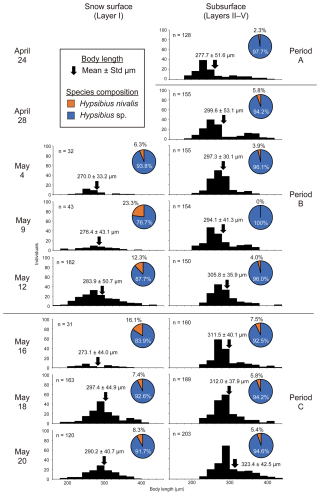
Figure 5Temporal changes in body size distribution and species composition of tardigrades living in snowpacks. Histogram of body length expressed in 20 µm increments. The species composition of tardigrades at each sampling time is displayed next to the histogram of body length.
The species composition of tardigrades differed between the snow surface and subsurface layers. Hypsibius sp. dominated both snow surface and subsurface layers throughout the season. H. nivalis consistently accounted for a higher proportion on the snow surface than that found in subsurface layers. Between Period B and the early stages of Period C, H. nivalis comprised 12.3 %–23.3 % of the tardigrade population on the snow surface and 0 %–7.5 % in the subsurface layers.
3.6 Temporal and vertical changes in solutes within the snowpack
Most chemical solutes in the snowpack were concentrated in the surface layer; however, each solute exhibited distinct temporal changes during the study period (Fig. 6). The concentrations of Na+, Cl−, SO, and NO were higher in Layers I and II than in the lower layers during Period A, particularly on 10 April, when snowfall occurred. Thereafter, no further increase in concentration was observed. The concentrations of NH, PO, and K+ remained low during Periods A and B and increased in surface layers I and II during Period C. This increase was particularly remarkable for algal-blooming snow surfaces. The concentration of K+ in the surface layer increased exponentially throughout Periods B to C in the algal-blooming snow. The concentrations of Mg2+ and Ca2+ in Period A were higher than those in the other periods, particularly in Layers I and II on 10 April. Although no significant variation was observed during Period B, their concentrations increased again in Period C in both algal-blooming and adjacent snow, particularly in Layer I.
3.7 Temporal changes in the amounts of insoluble materials on the snow surface
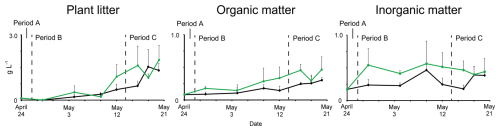
Measurements of the dry weights of insoluble materials on snow surfaces revealed that they changed temporally; however, their patterns differed among the types of material (Fig. 7). The total insoluble material on the snow surface was below the detection limit throughout Period A. Plant litter increased sharply from the middle of Period B (9 May) to the end of Period C (20 May). The amount of organic matter, excluding plant litter, increased from Period B (4 May) until the end of Period C. The amount of inorganic matter did not show an increasing trend, but fluctuated throughout Periods B and C. These trends were similar for both the algal-blooming and adjacent snow. The amounts of insoluble material collected on 28 April and 20 May showed that plant litter, organic matter, and inorganic matter were approximately twice as much in algal-blooming snow than in the adjacent snow (Table S1).
3.8 Chemical solutes in rainwater
Analysis of chemical solutes in rainwater collected from open and tree-covered areas revealed that nutrient concentrations increased after the budburst of beech trees in both areas (Figs. 8 and S2). The NH and K+ concentrations in rainwater collected from the open area increased by 55 % and 65 %, respectively, after the budburst. PO, K+, and Mg2+ concentrations in rainwater collected from tree-covered areas increased by 230 %, 326 %, and 15 %, respectively. The concentrations of all chemical solutes in rainwater measured in this study were significantly higher in tree-covered areas than in open areas, with the exception of NO and Ca2+ (Table S2).
3.9 Elution experiment of solutes from tree-derived litter
The elution experiment revealed that NH, PO, and K+ were eluted from the tree-derived litter on the snow surface into the water. The concentrations of Na+, Cl−, SO, and NO in the water did not change throughout the experiment. In contrast, the concentrations of NH, PO, and K+ increased at the beginning of immersion and remained high at 336 h (Fig. 8). Simultaneously, the concentrations of Mg2+ and Ca2+ gradually increased during 336 h immersion (Fig. S3).
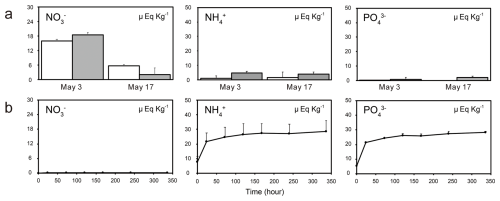
Figure 8Concentration of nutrients in (a) two rainwater samples collected during Periods B (3 May) and C (17 May) and (b) results of elution experiments. The nutrient concentrations in the rainwater samples collected from the open and tree-covered areas are shown using white and gray bars, respectively.
Temporal and vertical changes in the microbial community and environmental conditions indicated that microbial activity throughout the snowmelt season was distinct among the three periods (Periods A–C): the initial appearance of microbes (Period A), formation of algal blooms (Period B), and further blooming aided by the additional supply of nutrients (Period C). The following sections discuss the factors influencing the microbial activity during each period.
Despite the insulating properties of snow, studies have shown that air temperature fluctuations can affect snow temperatures down to depths of 20–30 cm, especially during frequent melt–freeze cycles.
4.1 Settlement of microbes into the snowpack (Period A)
The vertical distribution of microbes during Period A indicated that the biological activity within the snowpack did not enhance immediately after the onset of snowmelt. During Period A, the microbial abundance remained low. On 24 April, at the end of Period A, an increase in total microbial abundance was observed in Layers I–III (Fig. 4). In addition, the body size distribution of tardigrades showed no juveniles within the snowpack (Fig. 5). These findings suggest that, although microbes may already be present, their abundance does not increase substantially during the first month after snowmelt onset, and visible algal blooms do not yet occur. One possible reason for the lack of biological activity during this period is the number of freeze–thaw cycles in the surface layer. Although snow has strong insulating properties, temperature variations at the surface can influence the thermal regime of the snowpack up to depths of 20–30 cm, which corresponds to the sampling depth in this study, as observed in other regions and under various snow depth conditions (Zhang, 2005; Chen et al., 2013; Hirashima et al., 2015). During this period, the daily minimum temperature was below the freezing point, indicating that the snowpack had frozen. The snow algae and microinvertebrates observed in this study may have had difficulty remaining active under freezing conditions. A large proportion of vegetative cells in snow and ice algae are known to not recover after exposure to freezing (Hoham, 1975a); however, they can enhance freeze tolerance by accumulating polyunsaturated fatty acids and other compounds like osmolytes, which increase during cyst formation (Prochazkova et al., 2018; Procházková et al., 2019a; Ezzedine et al., 2023). Similarly, tardigrades found in snowpacks do not exhibit specific tolerance to freeze–thaw cycles, unlike some other tardigrades in glaciers that are well adapted to freeze–thaw cycles (Zawierucha et al., 2023). Compared with previous research, our findings indicate that Period A corresponds to a phase in which microbes are active but their growth in the upper snow layer is limited by the freezing air temperatures.
4.2 Emergence of algal blooms in snow (Period B)
The temporal changes in snow algae and chemical solutes observed during Period B suggest that the nitrate (NO) present in the upper snowpack was utilized for the growth and blooming of snow algae. During Period B, Types A and B algae initially increased between 24 and 28 April, coinciding with the appearance of green snow patches (Fig. 4). Among these, Type B algae exhibited exponential growth. In addition, nitrate concentrations remained high during this period. These results suggest that algal growth occurred through the utilization of available nutrients during this period. Previous research has shown that snow algae are present in environments with high NO concentrations (Hanzelová et al., 2018). The high concentration of NO in rainwater, the relationship of Type B algae with NO from the CCA analysis (Fig. S4), and the relationship of NO with sea salt-derived components, such as Na+ and Cl−, also indicate that the nitrogen used by algal growth in this region could be of atmospheric origin (Fukuzaki et al., 1999; Shinomiya et al., 2018).
The increase in microbial abundance during Period B suggests that the activity of other microbes was induced by algal blooms. The drastic increase in the population density of microinvertebrates and fungi coincided with the emergence of algal blooms in the snow (Fig. 4). For microinvertebrates, significant positive correlations were observed between the abundance of tardigrades and both Types A and B algae, as well as between rotifers and Type A algae (Fig. S5). The body size distribution of the tardigrades revealed no juveniles during this period, indicating absence of reproduction (Fig. 5). These results suggest that microinvertebrates migrate toward and assemble where food resources are available. This is consistent with the results of a previous study (Ono et al., 2021). The abundance of snow fungi Chi. nivalis showed a significant positive correlation with that of Type B algae (Fig. S5), suggesting that germination of the fungi was likely promoted by the presence of snow algae. A significant correlation between the abundance of Chi. nivalis and algae have also been reported from other snowpacks (Hoham et al., 1993; Yakimovich et al., 2020). These studies have suggested that algal exudates are nutritionally significant for fungal germination.
Algal growth at the end of this period was likely limited by nutrient depletion and the life cycle stages of the microinvertebrates changed during the same period. In the late Period B, abundance of all the microbes did not increase (Fig. 4), and nutrient concentrations during majority of this period were below 1.0 µEq kg−1 (Fig. 6), suggesting that their abundances had achieved their environmental carrying capacity due to depletion of nutrients. A previous study showed that snow algal growth reaches its maximum at a nitrate concentration that was more than 56 times higher than the concentration observed during Period B in this study (Broadwell et al., 2023). Furthermore, a decrease in Type B algae and a consistent increase in Type C algae were observed from late April to late May. Notably, the increase in Type C algae during the snowmelt season coincided with a decrease in NO, showing a significant negative correlation (Figs. 4, S5). These results suggest that Types B and C represent different life stages of the same algal species. Type C seems to be an elliptical cell before becoming dormant cells (Type B) of Chloromonas hindakii, Chloromonas miwae, and/or Chloromonas nivalis (Muramoto et al., 2008; Matsuzaki et al., 2019; Procházková et al., 2019a). Changes in algal life stages have been previously reported at the same study site (Muramoto et al., 2010), and nitrogen depletion has been suggested as a factor in the formation of dormant spores of snow algae (Hoham and Duval, 2001). These results also suggested that the microinvertebrates reproduced and hatched from the eggs at this time. The population density of microinvertebrates decreased in both algal-blooming and adjacent snows (Fig. 4), whereas tardigrade juveniles were observed on the snow surface during this period (Fig. 5). They likely laid eggs in the subsurface layers, and their egg-containing exuviae (previously reported in Ono et al., 2021) reached the snow surface as the snow melted, and eventual hatching occurred at the surface layer. The eggs of Hypsibius dujardini, the same genus inhabiting snowpacks, require 4–4.5 d to hatch (Gabriel et al., 2007). If this period also applies to tardigrades in snowpacks, it can be inferred that the observed increase in individuals results from the hatching of eggs laid in early May.
4.3 Enhancement of microbial growth by nutrients from canopy (Period C)
Changes in microbial abundance and snowpack conditions suggested that the budburst of beech trees triggered the regrowth of microbes in the snowpack during the late snowmelt season. The increase in Types A and B algae between 16 and 20 May coincided with increases in NH, PO, and K+, and a significant positive correlation was noted between their abundance and the concentration of chemical solutes (Figs. 4, S5). This suggests that algal growth in snow was promoted by the supply of nutrients to the snowpack. Previous studies have shown that NH and PO in snowpacks are limiting factors for algal growth (Jones, 1991; Suzuki and Watanabe, 2000; Hoham and Duval, 2001; Hanzelová et al., 2018). A laboratory experiment also showed that high Phosphorus availability promoted blooming of snow algae (Chloromonas rosae and Chloromonas typhlos; Almela et al., 2024).
Temporal changes in plant litter and nutrients and elution experiments on plant litter indicated that the increase in nutrient concentrations was due to the supply of litter and other insoluble particles deposited on the snow surface. Nutrients (NH and PO) increased on 18 May in both the algal-blooming and adjacent snow (Fig. 6). Because high concentrations of NH and PO were detected in the elution experiment, nutrients were likely supplied by plant litter deposited on the snow surface and utilized by snow algae. A recent study reported an increase in nutrients associated with budburst on Mt. Gassan in Yamagata Prefecture (Suzuki and Takeuchi, 2023). The abundance of snow algae and nutrients in snowpacks are known to increase with tree budburst (Hoham, 1976; Hoham et al., 2008). Especially in Hoham et al. (2008) reported from culture experiments using leachates of balsam fir needles that strain identity and leachate concentration affected the algal populations but did not alter the shape of population growth trajectories over time. In contrast, our study suggests that a large amount of nutrients may have leached from impurities (bud scales) derived from beech forests, potentially triggering a rapid algal growth. This indicates that the impacts on microbial communities can differ depending on plant species. Such effects could be further evaluated by analyzing the chemical composition of leachates from impurities originating from different tree species and by conducting algal culture experiments under these conditions.
The timing of the decrease in solar radiation below the tree canopy coincided with the budburst of beech trees and an increase in snow algae, suggesting that the budburst-associated reduction in solar radiation mitigates excessive light stress, enabling algae to remain active at the nutrient-rich snow surface. A decrease in solar radiation, the budburst of beech trees, and an increase in snow algae were observed on 15 and 16 May (Figs. 1, 3, and 4). The relationship between solar radiation and algal blooms has been previously described. Excessive light intensity can reduce the photosynthetic activity of snow algae and cause cellular damage (Remias et al., 2005; Procházková et al., 2023). Under these conditions, algal growth – as indicated by both increases in algal cell concentration – may be facilitated by the simultaneous availability of sufficient nutrients and moderate light levels (Suzuki and Takeuchi, 2023; Ono and Takeuchi, 2025). Based on these studies, it is possible that beech tree budburst contributes to enhanced algal growth on nutrient-rich snow surfaces, possibly by prolonging the period of favorable conditions for algal activity.
The vertical distribution of microinvertebrates during this period indicates increased microinvertebrate activity, similar to that of snow algae. Similar to snow algae, microinvertebrate populations increased again on 16 May, following the beech tree budburst (Fig. 4). However, unlike algae, they were concentrated in the subsurface layers. Their abundance and vertical distribution can be attributed to the availability of food resources and intensity of solar radiation. As observed during Period B, microinvertebrates were concentrated in the layers where algal cells were abundant. The high population density of tardigrades on the snow surface may have been caused by larval hatching. The intensity of solar radiation may still be above the limit that inhibits the activity of microinvertebrates within the snowpack, despite the budburst. Consequently, they are likely to be active within the snowpack rather than on the snow surface. Because snow algal abundance increased in the adjacent snow during this period, it is likely that algal growth in the adjacent snow facilitated a subsequent increase in microinvertebrate populations.
In this study, changes in radiation associated with tree leaf-out, such as increased longwave radiation as shown in previous study (Goodfellow and Barkham, 1974) to the snowpack or changes in shortwave absorption due to albedo reduction were not explicitly considered. These processes may also affect snow-ice microbes and should be examined in future studies.
4.4 Possible effects of changes in climate and forest phenology on microbial communities within snowpacks
The vertical distribution of microbes in the snow pits and cores indicates that microbes were active within the snowpack throughout the snowmelt season. Microbes in the snowpack were distributed from the surface to the depth of 30 cm during the snowmelt season (Fig. S1), and their vertical distribution varied according to the biota, with snow algae concentrated near the surface (0–3 cm deep) and microinvertebrates widely distributed within the snowpack (3–23 cm deep). These results are consistent with those of a previous study suggesting the presence of a Microbial Active Snow Surface (MASS) layer on a diurnal scale (Ono and Takeuchi, 2025). The results of the present study demonstrated that the MASS layer was maintained throughout the melting season.
While the intensity of solar radiation and nutrient concentrations on the snow surface have been considered as key factors determining the depth of the MASS layer, this study suggests that multiple factors influence their distribution throughout the melting season. One of such factors is snow depth (including maximum snow depth and air temperature), which determines the period during which microbes can migrate from the lower layers or from soil. Another important factor is the beech tree budburst, which influences nutrient supply and reduces solar radiation reaching the snow surface.
Future climate change is expected to alter the meteorological conditions, which could affect the biological activity within snowpacks. For example, global warming may lead to a decrease in maximum snow depth and earlier disappearance of snowpacks (Wakazuki et al., 2015; Kawase et al., 2020; Urban et al., 2023). Under such conditions, snowpacks may not persist until Period C. In addition, an increase in the mean temperature could cause an earlier onset of above-freezing conditions, thereby shortening the duration of Period A. However, this might not occur, as previous studies have suggested that the timing of snow disappearance would shift earlier at a faster rate than the onset of snowmelt (Urban et al., 2023). Nevertheless, earlier snowmelt could still increase the likelihood that snowpacks would disappear during Period A or B, potentially disrupting biological processes within the snowpack.
Additionally, phenological shifts due to rising temperatures, such as the earlier budburst of beech trees, may support earlier snow algal blooms. If budburst occurs earlier (Vitasse et al., 2009; Čufar et al., 2012), the transition from Period B to C, which is characterized by increased biological activity driven by nutrient input, may also occur earlier, potentially advancing the timing of peak microbial growth. In addition to phenological changes, shifts in the distribution of tree taxa have also been predicted in Japan (Matsui et al., 2004). These shifts may alter the chemical composition supplied from the elucidate of plant litter, significantly affecting the microbial activity within snowpacks in forested areas. During Period C, enhanced algal growth coincided with the timing of beech tree budburst, likely driven by an increase in nutrient input from fresh litter or throughfall. Therefore, future shifts in tree species composition could substantially alter the nutrient regimes in snowpacks, reshaping microbial dynamics.
Because these conditions vary across regions, future studies should examine not only algal growth and microbial biomass, but also their roles in driving biogeochemical processes such as carbon and nitrogen cycling within the snowpack. Considering the projected reductions in maximum snow depth and earlier dates of snow disappearance due to climate change, along with the expected changes in phenology, it is likely that the durations of Periods A and B will shorten. In contrast, the onset of Period C may occur earlier in response to an earlier budburst; however, its duration is expected to remain relatively unchanged. Changes in the durations of these periods and their interactions with the surrounding environment are expected not only to affect the abundance of organisms themselves, but also to influence biogeochemical processes driven by their biological activity. In future studies, it will be important to consider processes such as ion export during snowmelt and radiative feedback, including albedo changes. For example, as observed in Japanese snowpacks (Ohte et al., 2004; Osaka et al., 2016), melt-induced ion pulses could substantially influence nutrient availability and community dynamics. Future studies should consider constructing and testing models to evaluate the impacts of these snowpack physical processes on biological activity, as suggested by Costa et al. (2018, 2020). In addition to the effects of melt-induced ion pulses, future research should also take into account radiative feedback between trees, snow surface albedo, and snow-ice microbes (Conway et al., 1996; Aubry-Wake et al., 2022). Understanding these interactions will clarify how physical and biological processes jointly shape snowpack dynamics, nutrient availability, and biogeochemical cycling.
Temporal and vertical changes in the abundance of snow-ice microbes within the snowpack in the forested area of Mt. Gassan in Yamagata Prefecture were investigated, and the factors influencing these changes were discussed. This study demonstrates that microbial communities exhibit distinct seasonal dynamics in response to environmental conditions, with clear shifts in microbial activity between the defined periods of the melting season (Periods A–C). During Period A, microbial activity began, but remained limited because of sub-freezing air temperatures. Period B was characterized by an increase in microbial growth with increasing temperature; however, this growth was constrained by the depletion of available nutrients. In Period C, nutrient input after the budburst of trees, coupled with shading effects, increased microbial activity, particularly on the snow surface. These findings suggest that temporal changes in microbial activity in snowpacks are regulated by snow depth, temperature increase, and the phenology of trees above the snow surface, highlighting the complex interplay between physical and biological factors in shaping snow ecosystems. The results of pit and core sampling suggest that microbes in the snowpack are active not only on the snow surface but also within the snow, referred to as the Microbial Active Snow Surface (MASS) layer, throughout the snowmelt season, and that their abundance and vertical distribution change with nutrients and solar radiation. Further studies are required to clarify the effects of different tree species on biological activity and to better quantify the response of snow ecosystems to climate change.
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
The supplement related to this article is available online at https://doi.org/10.5194/tc-19-5983-2025-supplement.
Sampling for research: MO, KK, DS, FK, KW, and SU; manuscript conception: MO and NT; microscopy: MO; chlorophyll a: MO; chemical analysis: MO; and statistical analysis: MO. All authors have edited and reviewed the manuscript.
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors. Views expressed in the text are those of the authors and do not necessarily reflect the views of the publisher.
We would like to thank the filed campaign members of the current study. We also thank the two reviewers (Daniel Remias and an anonymous reviewer) and the editor (Francesco Avanzi) for helpful suggestions that improved this paper. We would like to thank Editage (https://www.editage.jp, last access: 2 May 2025) for English language editing.
This research has been supported by JSPS KAKENHI (grant nos. 19H01143, 20K21840, 20H00196, 21H03612, 22J11017, 22KJ0471, 24KJ0118, and 25K21373) and the Arctic Challenge for Sustainability II (ArCS II, Program grant no. JPMXD1420318865).
This paper was edited by Francesco Avanzi and reviewed by Daniel Remias and one anonymous referee.
Almela, P., Elser, J. J., Giersch, J. J., Hotaling, S., Rebbeck, V., and Hamilton, T. L.: Laboratory Experiments Suggest a Limited Impact of Increased Nitrogen Deposition on Snow Algae Blooms, Environ. Microbiol. Rep., 16, e70052, https://doi.org/10.1111/1758-2229.70052, 2024.
Amato, P., Hennebelle, R., Magand, O., Sancelme, M., Delort, A.-M., Barbante, C., Boutron, C., and Ferrari, C.: Bacterial characterization of the snow cover at Spitzberg, Svalbard: Bacterial characterization of an Arctic snow cover, FEMS Microbiology Ecology, 59, 255–264, https://doi.org/10.1111/j.1574-6941.2006.00198.x, 2007.
Amoroso, A., Domine, F., Esposito, G., Morin, S., Savarino, J., Nardino, M., Montagnoli, M., Bonneville, J.-M., Clement, J.-C., Ianniello, A., and Beine, H. J.: Microorganisms in Dry Polar Snow Are Involved in the Exchanges of Reactive Nitrogen Species with the Atmosphere, Environ. Sci. Technol., 44, 714–719, https://doi.org/10.1021/es9027309, 2010.
Aubry-Wake, C., Bertoncini, A., and Pomeroy, J. W.: Fire and Ice: The Impact of Wildfire-Affected Albedo and Irradiance on Glacier Melt, Earth's Future, 10, e2022EF002685, https://doi.org/10.1029/2022EF002685, 2022.
Broadwell, E. L. M., Pickford, R. E., Perkins, R. G., Sgouridis, F., and Williamson, C. J.: Adaptation versus plastic responses to temperature, light, and nitrate availability in cultured snow algal strains, FEMS Microbiology Ecology, 99, fiad088, https://doi.org/10.1093/femsec/fiad088, 2023.
Brown, R., Schuler, Vikhamar, Dagrun, Bulygina, O., Derksen, C., Luijus, K., Mudryk, L., Wang, L., and Yang, D.: Chapter 3. Arctic terrestrial snow cover, in: Snow, Water, Ice and Permafrost in the Arctic (SWIPA) 2017, Arctic Monitoring and Assessment Programme, Oslo, Norway, ISBN 978-82-7971-101-8, 2017.
Chen, X., Wei, W., and Liu, M.: Characteristics of temperature variation in seasonal snow in the Western Tianshan Mountains, China, Meteorological Applications, 20, 457–465, https://doi.org/10.1002/met.1308, 2013.
Conway, H., Gades, A., and Raymond, C. F.: Albedo of dirty snow during conditions of melt, Water Resources Research, 32, 1713–1718, https://doi.org/10.1029/96WR00712, 1996.
Costa, D., Pomeroy, J., and Wheater, H.: A numerical model for the simulation of snowpack solute dynamics to capture runoff ionic pulses during snowmelt: The PULSE model, Advances in Water Resources, 122, 37–48, https://doi.org/10.1016/j.advwatres.2018.09.008, 2018.
Costa, D., Baulch, H., Elliott, J., Pomeroy, J., and Wheater, H.: Modelling nutrient dynamics in cold agricultural catchments: A review, Environmental Modelling & Software, 124, 104586, https://doi.org/10.1016/j.envsoft.2019.104586, 2020.
Cruaud, P., Vigneron, A., Fradette, M., Dorea, C. C., Culley, A. I., Rodriguez, M. J., and Charette, S. J.: Annual bacterial community cycle in a seasonally ice-covered river reflects environmental and climatic conditions, Limnology & Oceanography, 65, https://doi.org/10.1002/lno.11130, 2020.
Čufar, K., De Luis, M., Saz, M. A., Črepinšek, Z., and Kajfež-Bogataj, L.: Temporal shifts in leaf phenology of beech (Fagus sylvatica) depend on elevation, Trees, 26, 1091–1100, https://doi.org/10.1007/s00468-012-0686-7, 2012.
Curl, H., Hardy, J. T., and Ellermeier, R.: Spectral Absorption of Solar Radiation in Alpine Snowfields, Ecology, 53, 1189–1194, https://doi.org/10.2307/1935433, 1972.
Degma, P.: Field and Laboratory Methods, in: Water Bears: The Biology of Tardigrades, vol. 2, edited by: Schill, R. O., Springer International Publishing, Cham, 349–369, https://doi.org/10.1007/978-3-319-95702-9_14, 2018.
Détain, A., Suzuki, H., Wijffels, R. H., Leborgne-Castel, N., and Hulatt, C. J.: Snow algae exhibit diverse motile behaviors and thermal responses, mBio, 16, e02954-24, https://doi.org/10.1128/mbio.02954-24, 2025.
Domine, F.: Should We Not Further Study the Impact of Microbial Activity on Snow and Polar Atmospheric Chemistry?, Microorganisms, 7, 260, https://doi.org/10.3390/microorganisms7080260, 2019.
Ezzedine, J. A., Uwizeye, C., Si Larbi, G., Villain, G., Louwagie, M., Schilling, M., Hagenmuller, P., Gallet, B., Stewart, A., Petroutsos, D., Devime, F., Salze, P., Liger, L., Jouhet, J., Dumont, M., Ravanel, S., Amato, A., Valay, J.-G., Jouneau, P.-H., Falconet, D., and Maréchal, E.: Adaptive traits of cysts of the snow alga Sanguina nivaloides unveiled by 3D subcellular imaging, Nat. Commun., 14, 7500, https://doi.org/10.1038/s41467-023-43030-7, 2023.
Felip, M., Sattler, B., Psenner, R., and Catalan, J.: Highly active microbial communities in the ice and snow cover of high mountain lakes, Appl. Environ. Microbiol., 61, 2394–2401, https://doi.org/10.1128/aem.61.6.2394-2401.1995, 1995.
Fukushima, H.: Studies on Cryophytes in Japan, Yokohama Municipal University, 43, 1–146, 1963.
Fukuzaki, N., Oshio, T., Noguchi, I., Matsumoto, M., Morisaki, S., Oohara, M., Tamaki, M., and Hiraki, T.: Spatial differences of chemical features of atmospheric deposition between rainy season and winter in the areas facing to the Japan sea, Japan, Chemosphere, 38, 411–423, https://doi.org/10.1016/S0045-6535(98)00189-1, 1999.
Gabriel, W. N., McNuff, R., Patel, S. K., Gregory, T. R., Jeck, W. R., Jones, C. D., and Goldstein, B.: The tardigrade Hypsibius dujardini, a new model for studying the evolution of development, Developmental Biology, 312, 545–559, https://doi.org/10.1016/j.ydbio.2007.09.055, 2007.
Goodfellow, S. and Barkham, J. P.: Spectral transmission curves for a beech (Fagus Sylvatica L.) CANOPY, Acta Botanica Neerlandica, 23, 225–230, https://doi.org/10.1111/j.1438-8677.1974.tb00940.x, 1974.
Grinde, B.: Vertical distribution of the snow alga Chlamydomonas nivalis (Chlorophyta, Volvocales), Polar Biol., 2, 159–162, https://doi.org/10.1007/BF00448965, 1983.
Hanzelová, M., Vido, J., Škvarenina, J., Nalevanková, P., and Perháčová, Z.: Microorganisms in summer snow patches in selected high mountain ranges of Slovakia, Biologia, 73, 1177–1186, https://doi.org/10.2478/s11756-018-0136-0, 2018.
Hao, C., Chen, T.-W., Wu, Y., Chang, L., and Wu, D.: Snow microhabitats provide food resources for winter-active Collembola, Soil Biology and Biochemistry, 143, 107731, https://doi.org/10.1016/j.soilbio.2020.107731, 2020.
Hirashima, H., Yamaguchi, S., Kosugi, K., Nemoto, M., Aoki, T., and Matoba, S.: Validation of the SNOWPACK model using snow pit observation data, Journal of the Japanese Society of Snow and Ice, 77, 5–16, https://doi.org/10.5331/seppyo.77.1_5, 2015.
Hoham, R. W.: Optimum Temperatures and Temperature Ranges for Growth of Snow Algae, Arctic and Alpine Research, 7, 13, https://doi.org/10.2307/1550094, 1975a.
Hoham, R. W.: The life history and ecology of the snow alga Chloromonas pichinchae (Chlorophyta, Volvocales), Phycologia, 14, 213–226, https://doi.org/10.2216/i0031-8884-14-4-213.1, 1975b.
Hoham, R. W.: The Effect of Coniferous Litter and Different Snow Meltwaters upon the Growth of Two Species of Snow Algae in Axenic Culture, Arctic and Alpine Research, 8, 377, https://doi.org/10.2307/1550440, 1976.
Hoham, R. W. and Duval, B.: Microbial ecology of snow and freshwater ice Snow Ecology, Cambridge: Cambridge University Press, ISBN 978-0521584838, 2001.
Hoham, R. W. and Remias, D.: Snow and Glacial Algae: A Review, J. Phycol., 56, 264–282, https://doi.org/10.1111/jpy.12952, 2020.
Hoham, R. W., Laursen, A. E., Clive, S. O., and Duval, B.: Snow algae and other microbes in several alpine areas in New England, 50th Eastern Snow Conf., 165–173, 1993.
Hoham, R. W., Mc Cay, T. S., Poirier, M., and Brazda Bell, T.: Balsam fir leaf litter extract stimulates growth of the green snow alga Chloromonas rosae var. psychrophila (Chlorophyta, Volvocales) from Whiteface Mountain, New York, nova_hedwigia, 86, 133–140, https://doi.org/10.1127/0029-5035/2008/0086-0133, 2008.
Holm-Hansen, O., Lorenzen, C. J., Holmes, R. W., and Strickland, J. D. H.: Fluorometric determination of chlorophyll, ICES J. Mar. Sci., 30, 3–15, 1965.
Irwin, N. A. T., Twynstra, C. S., Mathur, V., and Keeling, P. J.: The molecular phylogeny of Chionaster nivalis reveals a novel order of psychrophilic and globally distributed Tremellomycetes (Fungi, Basidiomycota), PLoS ONE, 16, e0247594, https://doi.org/10.1371/journal.pone.0247594, 2021.
Jones, H. G.: Snow chemistry and biological activity: a particular perspective on nutrient cycling, in: Seasonal Snowpacks, edited by: Davies, T. D., Tranter, M., and Jones, H. G., Springer Berlin Heidelberg, Berlin, Heidelberg, 173–228, https://doi.org/10.1007/978-3-642-75112-7_8, 1991.
Jones, H. G.: The ecology of snow-covered systems: a brief overview of nutrient cycling and life in the cold, Hydrol. Process., 13, 2135–2147, https://doi.org/10.1002/(SICI)1099-1085(199910)13:14/15%3C2135::AID-HYP862%3E3.0.CO;2-Y, 1999.
Kariya, Y.: Geomorphic processes at a snowpatch hollow on Gassan volcano, northern Japan, Permafrost Periglac. Process., 13, 107–116, https://doi.org/10.1002/ppp.412, 2002.
Kariya, Y.: Holocene landscape evolution of a nivation hollow on Gassan volcano, northern Japan, CATENA, 62, 57–76, https://doi.org/10.1016/j.catena.2005.02.004, 2005.
Kawase, H., Yamazaki, T., Sugimoto, S., Sasai, T., Ito, R., Hamada, T., Kuribayashi, M., Fujita, M., Murata, A., Nosaka, M., and Sasaki, H.: Changes in extremely heavy and light snow-cover winters due to global warming over high mountainous areas in central Japan, Prog. Earth Planet. Sci., 7, 10, https://doi.org/10.1186/s40645-020-0322-x, 2020.
Kawase, H., Fukui, S., Nosaka, M., Watanabe, S. I., Otomo, K., Murata, A., Murazaki, K., and Nakaegawa, T.: Historical regional climate changes in Japan in winter as assessed by a 5-km regional climate model with a land surface process, Prog. Earth Planet. Sci., 10, 7, https://doi.org/10.1186/s40645-023-00536-4, 2023.
Kawecka, B.: Ecology of snow algae, Polish Polar Research, 7, 407–415, 1986.
Larose, C., Dommergue, A., and Vogel, T.: The Dynamic Arctic Snow Pack: An Unexplored Environment for Microbial Diversity and Activity, Biology, 2, 317–330, https://doi.org/10.3390/biology2010317, 2013.
Matsui, T., Yagihashi, T., Nakaya, T., Tanaka, N., and Taoda, H.: Climatic controls on distribution of Fagus crenata forests in Japan, Journal of Vegetation Science, 15, 57–66, https://doi.org/10.1111/j.1654-1103.2004.tb02237.x, 2004.
Matsumoto, M., Hanneman, C., Camara, A. G., Krueger-Hadfield, S. A., Hamilton, T. L., and Kodner, R. B.: Hypothesized life cycle of the snow algae Chlainomonas sp. (Chlamydomonadales, Chlorophyta) from the Cascade Mountains, USA, Journal of Phycology, 60, 724–740, https://doi.org/10.1111/jpy.13454, 2024.
Matsuzaki, R., Nozaki, H., Takeuchi, N., Hara, Y., and Kawachi, M.: Taxonomic re-examination of “Chloromonas nivalis (Volvocales, Chlorophyceae) zygotes” from Japan and description of C. muramotoi sp. nov., PLoS ONE, 14, e0210986, https://doi.org/10.1371/journal.pone.0210986, 2019.
Matsuzaki, R., Takashima, Y., Suzuki, I., Kawachi, M., Nozaki, H., Nohara, S., and Degawa, Y.: The Enigmatic Snow Microorganism, Chionaster nivalis, Is Closely Related to Bartheletia paradoxa (Agaricomycotina, Basidiomycota), Microb. Environ., 36, https://doi.org/10.1264/jsme2.ME21011, 2021.
Matsuzaki, R., Nohara, S., Suzuki, I., and Kawachi, M.: Snow algae in Oze, Low Temperature Science, 80, 155–162, 2022.
Muramoto, K., Kato, S., Shitara, T., Hara, Y., and Nozaki, H.: Morphological and Genetic Variation in the Cosmopolitan Snow Alga Chloromonas nivalis (Volvocales, Chlorophyta) from Japanese Mountainous Area, Cytologia, 73, 91–96, https://doi.org/10.1508/cytologia.73.91, 2008.
Muramoto, K., Nakada, T., Shitara, T., Hara, Y., and Nozaki, H.: Re-examination of the snow algal species Chloromonas miwae (Fukushima) Muramoto et al. , comb. nov. (Volvocales, Chlorophyceae) from Japan, based on molecular phylogeny and cultured material, European Journal of Phycology, 45, 27–37, https://doi.org/10.1080/09670260903272607, 2010.
Nakanishi, H., Seto, K., Takeuchi, N., and Kagami, M.: Novel parasitic chytrids infecting snow algae in an alpine snow ecosystem in Japan, Front. Microbiol., 14, 1201230, https://doi.org/10.3389/fmicb.2023.1201230, 2023.
Negoro, H.: Seasonal Occurrence of the Apterous Wintr Stoneflis in the Mountaine and the High Mountaine Areas of Toyama Prefecture in Japan, Bulletin of the Toyama Science Museum, 32, 61–69, 2009.
Ohte, N., Sebestyen, S. D., Shanley, J. B., Doctor, D. H., Kendall, C., Wankel, S. D., and Boyer, E. W.: Tracing sources of nitrate in snowmelt runoff using a high-resolution isotopic technique, Geophysical Research Letters, 31, 2004GL020908, https://doi.org/10.1029/2004GL020908, 2004.
Ono, M. and Takeuchi, N.: The diel vertical migration of microbes within snowpacks driven by solar radiation and nutrients, Arctic, Antarctic, and Alpine Research, 57, 2460253, https://doi.org/10.1080/15230430.2025.2460253, 2025.
Ono, M., Takeuchi, N., and Zawierucha, K.: Snow algae blooms are beneficial for microinvertebrates assemblages (Tardigrada and Rotifera) on seasonal snow patches in Japan, Sci. Rep., 11, 5973, https://doi.org/10.1038/s41598-021-85462-5, 2021.
Ono, M., Takeuchi, N., and Zawierucha, K.: Description of a new species of Tardigrada Hypsibius nivalis sp. nov. and new phylogenetic line in Hypsibiidae from snow ecosystem in Japan, Sci. Rep., 12, 14995, https://doi.org/10.1038/s41598-022-19183-8, 2022.
Onuma, Y., Takeuchi, N., and Takeuchi, Y.: Temporal changes in snow algal abundance on surface snow in Tohkamachi, Japan, Bulletin of Glacier Research, 34, 21–31, https://doi.org/10.5331/bgr.16A02, 2016.
Osaka, K., Kugo, T., Komaki, N., Nakamura, T., Nishida, K., and Nagafuchi, O.: Atmospheric nitrate leached from small forested watersheds during rainfall events: Processes and quantitative evaluation, J. Geophys. Res.-Biogeo., 121, 2030–2048, https://doi.org/10.1002/2015JG003210, 2016.
Prochazkova, L., Remias, D., Rezanka, T., and Nedbalova, L.: Chloromonas nivalis subsp. tatrae, subsp. nov. (Chlamydomonadales, Chlorophyta): re-examination of a snow alga from the High Tatra Mountains (Slovakia), Fottea, 18, 1–18, https://doi.org/10.5507/fot.2017.010, 2018.
Procházková, L., Remias, D., Řezanka, T., and Nedbalová, L.: Ecophysiology of Chloromonas hindakii sp. nov. (Chlorophyceae), Causing Orange Snow Blooms at Different Light Conditions, Microorganisms, 7, 434, https://doi.org/10.3390/microorganisms7100434, 2019a.
Procházková, L., Leya, T., Křížková, H., and Nedbalová, L.: Sanguina nivaloides and Sanguina aurantia gen. et spp. nov. (Chlorophyta): the taxonomy, phylogeny, biogeography and ecology of two newly recognised algae causing red and orange snow, FEMS Microbiology Ecology, 95, fiz064, https://doi.org/10.1093/femsec/fiz064, 2019b.
Procházková, L., Remias, D., Holzinger, A., Řezanka, T., and Nedbalová, L.: Ecophysiological and ultrastructural characterisation of the circumpolar orange snow alga Sanguina aurantia compared to the cosmopolitan red snow alga Sanguina nivaloides (Chlorophyta), Polar Biol., 44, 105–117, https://doi.org/10.1007/s00300-020-02778-0, 2021.
Procházková, L., Matsuzaki, R., Řezanka, T., Nedbalová, L., and Remias, D.: The snow alga Chloromonas kaweckae sp. nov. (Volvocales, Chlorophyta) causes green surface blooms in the high tatras (Slovakia) and tolerates high irradiance, Journal of Phycology, 59, 236–248, https://doi.org/10.1111/jpy.13307, 2023.
R Core Team: R: A language and environment for statistical computing, R Foundation for Statistical Computing, Vienna, Austria, https://www.R-project.org/ (last access: 21 April 2024), 2024.
Raymond, B. B., Engstrom, C. B., and Quarmby, L. M.: The underlying green biciliate morphology of the orange snow alga Sanguina aurantia, Current Biology, 32, R68–R69, https://doi.org/10.1016/j.cub.2021.12.005, 2022.
Rea, M. E. and Dial, R. J.: An experimental assessment of active and passive dispersal of red snow algae on the Harding Icefield, southcentral Alaska, Arctic, Antarctic, and Alpine Research, 56, 2370905, https://doi.org/10.1080/15230430.2024.2370905, 2024.
Remias, D., Lütz-Meindl, U., and Lütz, C.: Photosynthesis, pigments and ultrastructure of the alpine snow alga Chlamydomonas nivalis, European Journal of Phycology, 40, 259–268, https://doi.org/10.1080/09670260500202148, 2005.
Remias, D., Wastian, H., Lütz, C., and Leya, T.: Insights into the biology and phylogeny of Chloromonas polyptera (Chlorophyta), an alga causing orange snow in Maritime Antarctica, Antarctic Science, 25, 648–656, https://doi.org/10.1017/S0954102013000060, 2013.
Segawa, T., Miyamoto, K., Ushida, K., Agata, K., Okada, N., and Kohshima, S.: Seasonal Change in Bacterial Flora and Biomass in Mountain Snow from the Tateyama Mountains, Japan, Analyzed by 16S rRNA Gene Sequencing and Real-Time PCR, Appl. Environ. Microbiol., 71, 123–130, https://doi.org/10.1128/AEM.71.1.123-130.2005, 2005.
Shinomiya, Y., Yamada, T., Hirai, K., Ono, K., Noguchi, S., Kubota, T., and Abe, T.: Monitoring the chemistry of rainwater and streamwater from a small forested watershed: Results in the Kamabuchi experimental watershed, Yamagata prefecture, Tohoku district, Japan between 2000 and 2014, Bulletin of FFPRI, 17, 273–300, https://doi.org/10.20756/ffpri.17.3_273, 2018.
Shoji, S. and Yoshimura, K.: Suppressed water availability in winter buds delays the bud burst of broad-leaved trees in a heavy snow forest, Tree Physiology, 45, tpaf048, https://doi.org/10.1093/treephys/tpaf048, 2025.
Suzuki, K. and Watanabe, Y.: Chemical changes of snowpack by microbiological activity and incubation experiment of Scenedesmus acuminatus, Journal of the Japanese Society of Snow and Ice, 62, 235–244, https://doi.org/10.5331/seppyo.62.235, 2000.
Suzuki, T. and Takeuchi, N.: Influence of vegetation on occurrence and color of snow algal blooms in Mt. Gassan, Yamagata Prefecture, Japan, Arctic, Antarctic, and Alpine Research, 55, 2173138, https://doi.org/10.1080/15230430.2023.2173138, 2023.
Tanabe, Y., Shitara, T., Kashino, Y., Hara, Y., and Kudoh, S.: Utilizing the Effective Xanthophyll Cycle for Blooming of Ochromonas smithii and O. itoi (Chrysophyceae) on the Snow Surface, PLoS ONE, 6, e14690, https://doi.org/10.1371/journal.pone.0014690, 2011.
Urban, G., Richterová, D., Kliegrová, S., and Zusková, I.: Reasons for shortening snow cover duration in the Western Sudetes in light of global climate change, Int. J. Climatol., 43, 5485–5511, https://doi.org/10.1002/joc.8157, 2023.
Vitasse, Y., Delzon, S., Dufrêne, E., Pontailler, J.-Y., Louvet, J.-M., Kremer, A., and Michalet, R.: Leaf phenology sensitivity to temperature in European trees: Do within-species populations exhibit similar responses?, Agricultural and Forest Meteorology, 149, 735–744, https://doi.org/10.1016/j.agrformet.2008.10.019, 2009.
Wakazuki, Y., Hara, M., Fujita, M., Suzuki, C., Ma, X., and Kimura, F.: Effect of climate change on the snow disappearance date in mountainous areas of central Japan, Hydrological Research Letters, 9, 20–26, https://doi.org/10.3178/hrl.9.20, 2015.
Yakimovich, K. M., Engstrom, C. B., and Quarmby, L. M.: Alpine Snow Algae Microbiome Diversity in the Coast Range of British Columbia, Front. Microbiol., 11, 1721, https://doi.org/10.3389/fmicb.2020.01721, 2020.
Zawierucha, K., Vecchi, M., Takeuchi, N., Ono, M., and Calhim, S.: Negative impact of freeze–thaw cycles on the survival of tardigrades, Ecological Indicators, 154, 110460, https://doi.org/10.1016/j.ecolind.2023.110460, 2023.
Zhang, T.: Influence of the seasonal snow cover on the ground thermal regime: An overview, Reviews of Geophysics, 43, 2004RG000157, https://doi.org/10.1029/2004RG000157, 2005.