the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Brief communication: Stream microbes preferentially respire young carbon within the ancient glacier dissolved organic carbon pool
Amy D. Holt
Jason B. Fellman
Anne M. Kellerman
Eran Hood
Samantha H. Bosman
Amy M. McKenna
Jeffery P. Chanton
Robert G. M. Spencer
Glaciers export ancient bioavailable dissolved organic carbon (DOC). Yet, the sources of organic carbon (OC) underpinning bioavailability are poorly constrained. We assessed the isotopic composition of respired OC from bioincubations of glacier DOC. Relative to bulk DOC, respired OC was younger (+4350 to 8940 years) and 13C enriched (+9.2 ‰ to 12.2 ‰), consistent with utilization of an in situ-produced microbial carbon source. These findings provide direct evidence that a hidden pool of young OC may underpin the high bioavailability of ancient glacier DOC.
- Article
(3465 KB) - Full-text XML
-
Supplement
(421 KB) - BibTeX
- EndNote
Glacier dissolved organic carbon (DOC) has been characterized as ancient and highly bioavailable to aquatic microbes (Hood et al., 2009). Hence, glacier runoff is thought to stimulate downstream heterotrophy and ultimately release relic carbon to the atmosphere (Hood et al., 2009). The glacier DOC pool is derived from a mixture of organic carbon (OC) of various provenances, chemical compositions, and ages, including anthropogenic aerosols, soil- and plant-derived material (subglacial or windblown), and in situ microbial production either on or beneath the glacier (Hood et al., 2009; Stubbins et al., 2012; Spencer et al., 2014; Musilova et al., 2017; Smith et al., 2017; Behnke et al., 2020; Holt et al., 2021, 2023, 2024). However, it remains unclear which OC sources are responsible for the high bioavailability of glacier DOC. Elucidating the source of bioavailable organics is essential for understanding the fate of glacier-derived DOC and how this pool may change with glacier recession.
Observations of the source of the bioavailable fraction of glacier DOC are ambiguous, since past work suggests that either an aged or a young component and source of DOC may be most bioavailable. Macroinvertebrates in glacier-fed streams and forelands have been found to be 14C depleted (i.e., old; Hågvar and Ohlson, 2013; Fellman et al., 2015), indicating that aged OC is assimilated into food webs and thus perhaps underpins glacier DOC bioavailability. Furthermore, the percentage of bioavailable DOC has been negatively correlated with Δ14C-DOC, suggesting that aged DOC may be most bioavailable (e.g., Hood et al., 2009). However, this relationship has often been observed in watersheds covering broad gradients of glacier influence, where inputs of non-glacial DOC confound precise identification of the source(s) of bioavailable OC within the glacier DOC pool. Recent molecular-level assessment of supraglacial and outflow dissolved organic matter (DOM) composition has shown that the relative abundance (RA) of bioavailable, aliphatic compounds increases as the DOC pool becomes younger and δ13C enriched (Holt et al., 2023, 2024), further complicating whether the aged component of DOC contributes to the bioavailable fraction. Similarly, the concentration of bioavailable compounds (e.g., carbohydrates and amino acids) has been shown to increase with in situ microbial OC production on the glacier surface (Musilova et al., 2017). Together, these recent studies suggest that young in situ-derived OC could underpin the high bioavailability of DOC in supraglacial ecosystems and glacier outflows.
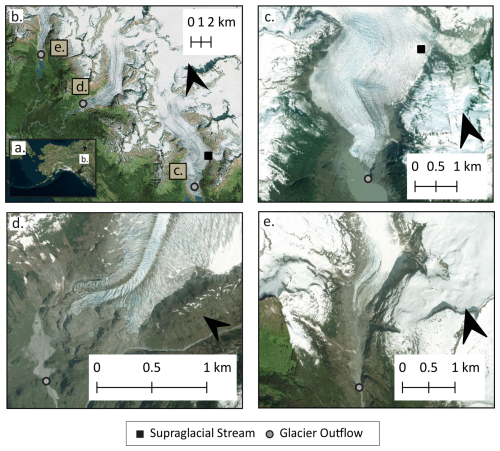
Here we investigate the age and potential sources of the respired fraction of DOC in a supraglacial stream and three glacier outflows in the Alaska Coast Mountains (Fig. 1). We quantified the carbon isotopic (δ13C and Δ14C – i.e., source and age) signature of bulk glacier DOC and, for the first time, respired OC (as CO2) using respiratory carbon recovery system (RCRS) experiments, which allow the CO2 produced by microbial respiration of DOC to be captured and its isotopic signature (δ13C-CO2 and Δ14C-CO2) assessed (McCallister et al., 2006). Isotopic signatures were used in conjunction with molecular-level data derived from 21 T Fourier transform ion cyclotron resonance mass spectrometry (FT-ICR MS) to evaluate the interplay between DOM composition and the age and source of respired OC. We hypothesized that respiratory CO2 would be isotopically younger and 13C enriched compared to bulk DOC, consistent with the notion that in situ microbial production within glacier ecosystems fuels microbial heterotrophy (e.g., Musilova et al., 2017; Smith et al., 2017; McCrimmon et al., 2018). Ultimately, our findings provide novel insights into glacier DOC source and bioavailability, with ramifications for our understanding of how the OC subsidy that glaciers provide to downstream ecosystems may be altered by continued glacier retreat.
2.1 Study sites and sample collection
Water samples were collected between 11 and 14 July 2022 from three glacierized watersheds in coastal southeast Alaska (Fig. 1). The study area is situated within the Juneau Icefield in coastal temperate rainforest and has a cool (annual mean temperature 5.6 °C), maritime climate, with the majority of precipitation falling in autumn and winter (Behnke et al., 2020). The geology of the upper watersheds, where the glaciers are found, is dominated by Tertiary–Cretaceous-aged, foliated tonalite sill of the coast plutonic complex (Wilson et al., 2015). These glaciers are well studied and are known to have discharge regimes and biogeochemical characteristics representative of glacial systems throughout the Gulf of Alaska. These glacier rivers are highly turbid, with low summer temperatures (<5 °C) and oligotrophic conditions (Hood and Scott, 2008; Fellman et al., 2014; Spencer et al., 2014). Based on past observations of conductivity, at the time of sampling, water residence times within the glaciers were short (∼hours), and subglacial drainage was relatively efficient, with the vast majority of meltwater having a supraglacial origin (Spencer et al., 2014).
Water samples were collected from the surface and outflow of Mendenhall Glacier, as well as downstream () of the terminus of both Eagle and Herbert glaciers. Between the glacier terminus and sampling sites, glacier outflow rivers flowed through recently deglaciated terrain (i.e., barren ground of cobble, gravel, and glacier silt, with few colonizer plants), and thus there was limited potential for OC inputs from vascular plants and soil organic matter. At Mendenhall, outflow sampling was conducted on a rock/silt bar<100 m from the glacier outflow. Water here is extremely turbulent and flows rapidly into Mendenhall Lake. As such, there is limited influence of the lake water, and sampling is representative of water exiting the glacier. The supraglacial sample was collected from a small (<1 m across) flowing supraglacial stream on the bare ice surface accessed by helicopter, ∼3 km upslope of the glacier terminus.
At each site, water was immediately filtered to 0.45 µm (Geotech Polyethersulfone dispos-a-filter™ capsule), acidified to pH 2 (10 M HCl), and stored (<2 weeks) at −20 °C in the dark until further processing. Filtrate was collected for RCRS experiments and for analysis of DOC concentration, carbon isotopes of DOC, and molecular-level composition. Samples were stored in 125 mL (DOC) or 1 L (other analyses) polycarbonate bottles. Additionally, at each site, water was also filtered to 1.6 µm using pre-combusted GF/A filters, stored in a 500 mL polycarbonate bottle at 4 °C in the dark, and used in the preparation of the RCRS experiment inocula.
2.2 Respiratory carbon recovery system experiments
The RCRS experiments were conducted following established methodology (McCallister et al., 2006). For each site, 0.45 µm filtrate was decanted into a 1 L acid-washed, pre-combusted serum bottle and then crimp-sealed, ensuring the bottle was gastight. Samples were then sparged in the dark with ultra-high-purity He (2 h, 0.08 L min−1) to strip dissolved inorganic carbon (DIC) from solution. Subsequently, samples were neutralized (∼pH 7) with DIC-free NaOH and reoxygenated with ultra-high-purity O2 (0.5 h, 0.08 L min−1) until >20.95 % O2 air saturation, as monitored by a PreSen Fibox O2 needle probe.
Inocula were prepared from the 1.6 µm filtrate. Aliquots from each site were mixed in equal proportion to form a composite inoculum, an approach used in laboratory bioassays because it controls for the potential influence of site-specific differences in bacterial community composition on DOC removal. For each experiment, 42 mL of composite inocula was filtered through a 0.2 µm Whatman® polycarbonate Nuclepore Track-EtchMembrane filter using a pre-cleaned glass filter tower. Using a needle and syringe, DIC-free incubation water was extracted from the serum bottle and used to resuspend microbes harvested on the filter. This process acts to limit the amount of DIC entering the DIC-free, closed-system experiment. The resuspended microbial community (∼2.5 mL) was injected into the serum bottle. Samples were incubated at 22 °C in the dark for 28 d, as is standard for past DOC and glacier DOC bioincubations (e.g., Hood et al., 2009; Holt et al., 2023).
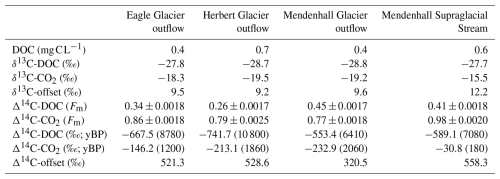
Table 1Dissolved organic carbon (DOC) concentrations and carbon isotopic signatures of DOC and respiratory CO2, along with the offset between measured carbon isotopic values.
Following incubation, samples were acidified to pH 2 using DIC-free HCl. Evolved CO2 from microbial respiration of DOC was sparged from solution (2 h, 0.08 L min−1) with ultra-high-purity He and trapped cryogenically (liquid N2) on a vacuum line. The CO2 was then purified through a series of cryogenic traps before being quantified and isolated in a pre-combusted (550 °C, 5 h) break seal (McCallister et al., 2006).
2.3 Dissolved organic carbon quantification and carbon isotopic analyses
Concentrations of DOC were measured on a Shimadzu TOC-LCPH analyzer following standard methods (e.g., Holt et al., 2024, and references therein). Before analysis, samples were sparged with air for 5 min at a flow rate of 0.08 L min−1 to remove DIC from solution. Measured concentrations are based on 3 of 7 replicate injections with a coefficient of variance of <2 %. 13C and 14C were measured via isotope ratio mass spectrometry (IRMS) and accelerator mass spectrometry, respectively, at Woods Hole Oceanographic Institution. For DOC isotopes, samples were UV-oxidized, and the resultant CO2 was cryogenically trapped for analysis. δ13C values measured by IRMS have a typical precision of <0.2 ‰ (Xu et al., 2021). Estimates of the contributions from radiocarbon dead (−1000 ‰) versus modern (95 % of 14C concentration in 1950 of NBS Oxalic Acid I normalized to ) OC were calculated from fraction modern (Fm) values (Table 1), where the percentage of radiocarbon dead material was determined as 1−Fm (Stubbins et al., 2012). Measurement error in Fm ranged from 0.0017–0.0025 (Table 1), making little quantitative difference to calculated values (i.e., ‰ and years before present (yBP)) and estimated source contributions.
2.4 Molecular-level analysis of dissolved organic matter
Water samples were solid-phase-extracted (100 mg Bond Elut PPL cartridges) and analyzed via negative-ion electrospray ionization 21 T FT-ICR MS using standard methods (e.g., Holt et al., 2021, 2023, 2024, and references therein). In brief, the volume extracted was adjusted depending on the sample DOC concentration to achieve a target eluent concentration of 40 µg C L−1. Cartridges were eluted with 1 mL of methanol. Mass spectra were formed from 100 scans conditionally co-added and phase-corrected. Spectra were internally calibrated in Predator analysis using the “walking calibration”. Peaks with signal-to-noise ratios greater than the baseline plus 6σ were exported to a peak list. Elemental composition was assigned to peaks within the bounds () using PetroOrg©. Assigned molecular formulae were classed by heteroatom content (CHO, CHON, CHOS, and CHONS) and grouped into commonly used, operational compound classes using the modified aromaticity index (AImod) and elemental stoichiometry (Holt et al., 2021, and references therein): condensed aromatics and polyphenolic (AImod values >0.67 and of 0.5–0.67, respectively), highly unsaturated and phenolic (HUP; AImod of <0.5 and ), and aliphatics ( and ). Sugar-like compounds ( and ) were also identified; these made up ≤0.2 % RA of all samples and thus are not discussed further.
3.1 Carbon isotopic and molecular composition of glacier dissolved organic matter
Concentrations of DOC ranged from 0.4–0.7 mg C L−1, consistent with published values for these sites (Table 1; Hood et al., 2009; Stubbins et al., 2012; Spencer et al., 2014; Behnke et al., 2020). Supraglacial stream and outflow DOC was ancient (median Δ14C-DOC −628.3 ‰, range 10 800–6410 yBP; Table 1, Fig. 2), as is typical of DOC derived from these and other glacier ecosystems (Hood et al., 2009; Stubbins et al., 2012; Holt et al., 2024). There was minimal variability in δ13C-DOC values, which ranged from −28.8 to −27.7 ‰ (Table 1, Fig. 2), overlapping with those reported previously for glacier outflow DOC (e.g., Hood et al., 2009; Holt et al., 2024).
At the molecular level, all sites were dominated by CHO-only (74.6 %–79.5 % RA) and HUP (58.4 %–73.0 % RA) formulae, as is typical of DOM in glacial and non-glacial aquatic ecosystems (Table S1 in the Supplement and Fig. 3; e.g., Behnke et al., 2020). Heteroatom-containing (CHON, CHOS, and CHONS) and aliphatic formulae were abundant in the supraglacial stream and outflows (median 24.3 % and 22.1 % RA, respectively), and condensed aromatic and polyphenolic compounds were a minor fraction of DOM composition (5.9 %–8.7 % RA; Table S1 and Fig. 3). This is consistent with previous molecular-level assessments from these sites and other glacier ecosystems, where glacier DOM is characterized as relatively heteroatom enriched and low in aromaticity compared to rivers with no to low glacier inputs (e.g., Behnke et al., 2020; Holt et al., 2024).
3.2 Composition and source of glacier dissolved organic matter
The isotopic and molecular-level composition of the supraglacial stream and glacier outflows was consistent with OC derived from a mixture of sources. Past studies of southeast Alaskan glaciers suggest that a substantial fraction of glacier DOC is derived from anthropogenic aerosols (Stubbins et al., 2012; Spencer et al., 2014; Holt et al., 2024). Based on simple mixing of radiocarbon dead (i.e., a purely fossil fuel source) and modern OC, of DOC across our study sites could have been derived from fossil fuel combustion byproducts, in line with past estimates (Table 1; Stubbins et al., 2012). This material was consistent with the presence of condensed aromatic compounds on the glacier surface and in the outflows and may have also contributed to the observed aliphatic-rich composition, especially if photodegraded (Table S1 and Fig. 3; Holt et al., 2021). In glacier outflows, a component of aged OC may also originate from subglacial material (e.g., overridden soils enriched in aromatic moieties) or DOC aged during glacier ice formation (Hood et al., 2009; Stubbins et al., 2012). Nonetheless, given the compositional similarity between supraglacial and outflow DOM (Figs. 2 and 3) and the residence time of glacier ice in these catchments (∼300 yBP; Stubbins et al., 2012), these contributions are likely marginal.
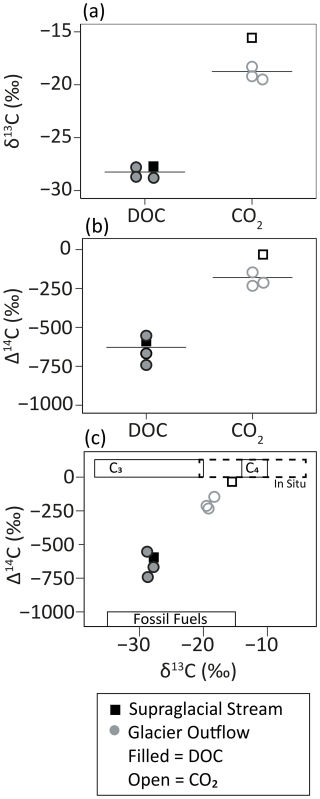
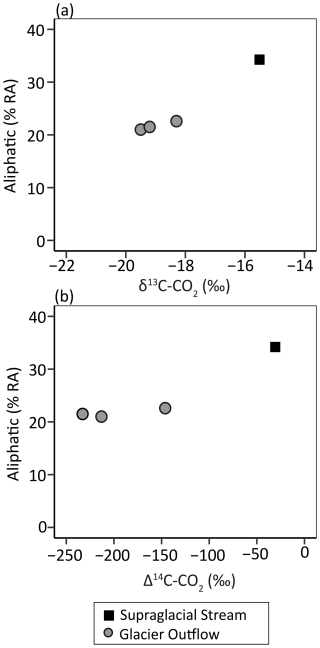
Figure 2The (a) δ13C and (b) Δ14C signature of DOC and respiratory CO2 and (c) the respective signatures in relation to possible endmember sources. Samples are colored and shaped according to their type (outflow and supraglacial – grey circle and black square, respectively). Filled and open symbols represent DOC and CO2, respectively. (a, b) The solid line represents the median value. (c) Boxes represent endmember sources: C3 plants (Kohn, 2010), C4 plants (Cerling et al., 1997), fossil fuels (Wang et al., 2022), and in situ production taken from the range published for cyanobacteria (Schmidt et al., 2022) and sea ice algae (Hobson and Welch, 1992; McMahon et al., 2006). A description of C3 and C4 plants is found in the Supplement.
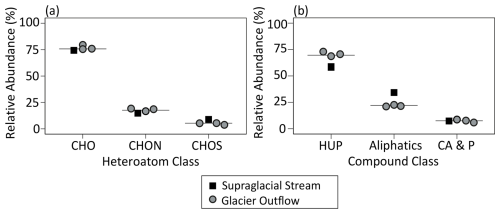
Figure 3Molecular-level composition of dissolved organic matter (DOM) before bioincubation. Plots show the percent relative abundance (RA) of (a) heteroatom and (b) compound classes. Samples are colored and shaped according to type (outflow and supraglacial – grey circle and black square, respectively). The solid line represents the median value. HUP: highly unsaturated and phenolic. CA and P: condensed aromatic and polyphenolic.
A sizable portion of DOC was also derived from younger or modern (up to 45 % of DOC) OC sources, rich in aliphatic moieties. This likely included in situ microbial production (e.g., by ice algae or cyanobacteria) on the glacier surface, which is known to produce aliphatic compounds (Musilova et al., 2017), and potentially vegetation and soil organic matter. Soil and vegetation OC could have been sourced from lateral inputs or atmospheric deposition (Spencer et al., 2014; Holt et al., 2024). This organic matter may have been the source of the relatively small fraction of polyphenolic compounds (<7.0 % RA) within the glacier DOM pools (Table S1 and Fig. 3) and potentially saturated compounds, particularly if photodegraded (Holt et al., 2021).
3.3 Isotopic composition of respiratory carbon and relation to molecular composition of dissolved organic matter
The RCRS experiments using supraglacial and glacier outflow DOC produced respiratory CO2 (0.2–0.3 mg C; Table S2 in the Supplement) with δ13C and Δ14C values ranging from −19.5 ‰ to −15.5 ‰ and −232.9 ‰ to −30.8 ‰ (2060–180 yBP), respectively (Table 1). These values were positively offset (δ13C and Δ14C-CO2 median +9.6 ‰ and +525.0 ‰, respectively) from the isotopic values of the initial DOC pool (Fig. 2). Hence, during the incubation, microbial community respiration was predominately fueled by organic compounds within the DOC pool that were relatively enriched in 13C and 14C (i.e., younger) compared to bulk DOC. Enriched δ13C-CO2 values may be associated with an increased percent RA of aliphatic compounds in the initial DOM pool and, in the case of the supraglacial stream, appear to coincide with substantially younger CO2 (Fig. 4). Given the small dataset (n=4), it is unclear whether this represents a compositional trend. However, the data suggest that the 13C- and 14C-enriched source respired during the incubations could contain a greater RA of aliphatic compounds.
3.4 Sources of respired glacier dissolved organic carbon
The RCRS experiments demonstrated that microbial respiration was fueled by younger and 13C-enriched OC compared to the bulk DOC pool (Fig. 2). Although isotopic signatures of OC endmembers are poorly constrained for glaciers, the δ13C values of respiratory CO2 (−19.5 to −15.5 ‰) overlapped with those of sea ice algae (−21 ‰ to −16 ‰) and cyanobacteria (−16 ‰ to −6 ‰), providing a line of evidence to suggest that bioavailable OC could be derived from in situ microbial sources (Table 1 and Fig. 2; Hobson and Welch, 1992; McMahon et al., 2006; Schmidt et al., 2022). Similarly, total OC concentrations of cryoconite hole sediments have been shown to positively correlate with δ13C, where enriched values ( ‰ to −10 ‰) were postulated to reflect greater relative contributions of in situ-produced OC compared to atmospherically deposited OC (Schmidt et al., 2022). Since microbial production on the glacier surface would be associated with enriched δ13C signatures (e.g., Schmidt et al., 2022; Holt et al., 2024), the respired 13C-enriched OC that we observed may be largely derived from in situ production (e.g., snow, ice algae, or cyanobacteria). Furthermore, the supraglacial stream had the most aliphatic DOM and the most 13C- and 14C-enriched CO2 (Figs. 2–4). This is consistent with autochthonously sourced aliphatic organic matter (Musilova et al., 2017; Holt et al., 2024) and further suggests that respired organics were derived from in situ production. Additionally, the relative similarity in molecular and isotopic composition (DOC and respiratory CO2) between the supraglacial stream and the glacier outflow samples suggests that a portion of the bioavailable OC exported from these glaciers originates from recent in situ production on the surface rather than from subglacial sources (Table 1, Figs. 2 and 3). However, subglacial sources such as paleosols and microbial chemotrophic processes could still contribute OC to the outflow DOM pool. Our findings are in line with past studies highlighting that microbes in cryoconite hole sediments fix atmospheric CO2, and that recently fixed OC compounds on glacier surfaces support microbial heterotrophy (Musilova et al., 2017; Smith et al., 2017; McCrimmon et al., 2018). It is conceivable that an alternative source of young 13C-enriched OC could have fueled respiration. This could include modern C4 plant material (δ13C −14 ‰ to −10 ‰; see the Supplement for a description of C3 and C4 plants), for example aliphatic moieties from combustion byproducts or pollen (Fig. 2; Cerling et al., 1997; Holt et al., 2021, 2023). Nonetheless, the dominance of C3 vegetation (δ13C −37 ‰ to −20 ‰; Kohn, 2010) in the coastal temperate rainforest of southeast Alaska makes a C4 source unlikely and instead supports in situ microbial production on the glacier surface as an important source of young bioavailable OC to the glacier DOC pools.
Despite overall shifts to younger isotopic signatures relative to bulk glacier DOC (median offset +525.0 ‰), the evolved CO2 produced from microbial respiration was still slightly aged (−232.9 ‰ to −30.8 ‰, 2060 to 180 yBP; Table 1 and Fig. 2). This aged OC could have been sourced from radiocarbon dead material like fossil fuel combustion byproducts. Fossil fuel sources and their photodegraded byproducts are known to contain aliphatic compounds (Holt et al., 2021) and therefore could be a source of bioavailable OC respired during the incubation period. Fossil fuel sources are on average more 13C depleted compared to the isotopic composition of respiratory CO2 (Table 1; Wang et al., 2022). Therefore, if fossil fuels contributed 13C-enriched OC, this could have originated from a 13C-enriched fossil fuel source; compounds within the sources' OC pool that are relatively 13C enriched compared to the bulk; or photodegradation of fossil-fuel-derived compounds, which is known to result in 13C enrichment (Spencer et al., 2009). In outflows, ancient DOC compounds could also be derived from aged ice-locked and subglacial sources (e.g., paleosols and microbial chemotrophic processes), although supporting data for these sources are yet to be observed for Alaskan glaciers (Stubbins et al., 2012; Spencer et al., 2014). The combined 13C enrichment and relatively young age of respiratory CO2 (Table 1) confirm that any aged, 13C-depleted source likely made up a small fraction of respired DOC during the incubations. It remains unclear how stream microbes utilize different OC compounds within the 28 d incubation period and whether or not different sources, including ancient OC, are preferentially metabolized or potentially prime respiration of less favorable OC sources. Additionally, since the samples were collected during peak melt (July) from a small number (n=3) of proximate glaciers in southeast Alaska, it is yet to be quantified whether temporal shifts and regional differences in bulk DOM composition (Spencer et al., 2014; Holt et al., 2024) impact the source and magnitude of respired OC. Given these bioincubation experiments were conducted under uniform conditions (i.e., uniform pH, temperature, and O2 saturation), comparisons of the data between them are appropriate, but it is unclear how in situ physiochemical properties and geochemistry may effect DOC metabolism and fate in the real world. Moreover, microbial carbon utilization and DOC bioavailability in the environment are a function of both respiration and biomass production, the latter of which was not assessed here. Ultimately, DOC from glacier ecosystems globally is exported to a range of aquatic environments (e.g., proglacial lakes, lower reaches of rivers, fjords, estuaries, and nearshore marine environments) with variable transit times, residence times, and environmental conditions. How these variations affect which glacier OC sources are used by the microbial food web is yet to be determined. That said, this study provides the first quantitative glimpse into the carbon sources underpinning the bioavailability of the ancient glacier DOC pool using a direct experimental approach.
Glaciers globally have been described as a source of ancient bioavailable DOC to downstream and marine food webs (e.g., Hood et al., 2009; Fellman et al., 2015; Holt et al., 2024). Our study in the Alaska Coast Mountains provides direct evidence that despite being aged, the high bioavailability of glacier DOC may be predominantly underpinned by younger OC, likely sourced from in situ microbial production on the glacier surface. Though yet to be quantified, this in situ source of carbon could be critical for stream heterotrophy across deglaciating watersheds and could currently subsidize food webs that support socioeconomically important fisheries (e.g., in the Gulf of Alaska and broadly the northern Pacific and Atlantic). Despite the dominance of young OC, we show respiratory CO2 was slightly aged (180–2060 yBP) across the study sites, demonstrating that glacier runoff mobilizes some relic carbon that can be assimilated into food webs and released to the atmosphere (Hood et al., 2009; Fellman et al., 2015). Nonetheless, if predominately modern, in situ production underpins OC bioavailability across glaciers, as suggested by this dataset, then microbial metabolism of glacier-derived DOC in downstream ecosystems globally may be primarily cycling contemporary material, rather than largely a microbially mediated release of aged carbon to the atmosphere. Importantly, as glaciers continue to recede, the glacier-derived DOC flux declines, and stream physicochemical conditions become more conducive to microbial production (e.g., Hood et al., 2015; Kohler et al., 2024), the source of this bioavailable, modern OC will likely switch from glacier-derived (e.g., glacier surface algal production) to instream sources.
Raw FT-ICR MS spectra files, calibrated peak lists, and assigned elemental composition data are available in the Open Science Framework (OSF; https://osf.io/4m2kx/, last access: 28 July 2025) repository under the following DOI: https://doi.org/10.17605/OSF.IO/4M2KX (Holt and Spencer, 2024).
The supplement related to this article is available online at https://doi.org/10.5194/tc-19-2769-2025-supplement.
RGMS: research conceptualization; ADH, JBF, EH, and RGMS: funding acquisition and/or resources; ADH, JBF, and EH: fieldwork; ADH: RCRS experiments with support from AMK, SHB, and JPC; ADH and AMM: laboratory analyses; ADH: data analysis and interpretation with support and supervision from JBF, EH, and RGMS; ADH: writing of the original draft with all authors contributing to writing review and editing and significant input from JBF, EH, and RGMS.
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
Jason B. Fellman and Eran Hood were supported by the National Science Foundation (NSF) Alaska EPSCoR Program (OIA-1757348) and Division of Earth Sciences (EAR-2227821). The 21 T FT-ICR MS analysis was performed in the Ion Cyclotron Resonance User Facility, National High Magnetic Field Laboratory, Florida, USA, which is supported by the NSF Division of Chemistry and Division of Materials Research through DMR 16-44779 and DMR 2128556. Amy D. Holt and Robert G. M. Spencer are extremely grateful to the Winchester Foundation and the International Association of Geochemistry for research support. NSF supported this research through an INTERN award to Amy D. Holt through the Arctic Great Rivers Observatory award (Robert G. M. Spencer: 1914081).
This research has been supported by the Office of Polar Programs (grant no. 1914081), the Division of Chemistry (grant no. 16-44779), the Division of Materials Research (grant no. 2128556), the Division of Earth Sciences (grant no. EAR-2227821), and the National Science Foundation (NSF) Alaska EPSCoR Program (grant no. OIA-1757348).
This paper was edited by Elizabeth Bagshaw and reviewed by two anonymous referees.
Behnke, M. I., Stubbins, A., Fellman, J. B., Hood, E., Dittmar, T., and Spencer, R. G.: Dissolved organic matter sources in glacierized watersheds delineated through compositional and carbon isotopic modeling, Limnol. Oceanogr., 66, 438–451, 2020.
Cerling, T. E., Harris, J. M., MacFadden, B. J., Leakey, M. G., Quade, J., Eisenmann, V., and Ehleringer, J. R.: Global vegetation change through the Miocene/Pliocene boundary, Nature, 389, 153–158, 1997.
Fellman, J. B., Nagorski, S., Pyare, S., Vermilyea, A. W., Scott, D., and Hood, E.: Stream temperature response to variable glacier coverage in coastal watersheds of Southeast Alaska, Hydrol. Process., 28, 2062–2073, 2014.
Fellman, J. B., Hood, E., Raymond, P. A., Hudson, J., Bozeman, M., and Arimitsu, M.: Evidence for the assimilation of ancient glacier organic carbon in a proglacial stream food web, Limnol. Oceanogr., 60, 1118–1128, 2015.
Hågvar, S. and Ohlson, M.: Ancient carbon from a melting glacier gives high 14 C age in living pioneer invertebrates, Sci. Rep., 3, 1–4, 2013.
Hobson, K. A. and Welch, H. E.: Determination of trophic relationships within a high Arctic marine food web using δ13C and δ15N analysis, Mar. Ecol. Prog. Ser., 84, 9–18, 1992.
Holt, A. D. and Spencer, R.: P19289_Stream Microbes Preferentially Utilize Young Carbon within the Ancient Glacier Dissolved Organic Carbon Pool, OSF [data set], https://doi.org/10.17605/OSF.IO/4M2KX, 2024.
Holt, A. D., Kellerman, A. M., Li, W., Stubbins, A., Wagner, S., McKenna, A., Fellman, J., Hood, E., and Spencer, R. G.: Assessing the Role of Photochemistry in Driving the Composition of Dissolved Organic Matter in Glacier Runoff, J. Geophys. Res.-Biogeo., 126, e2021JG006516, https://doi.org/10.1029/2021JG006516, 2021.
Holt, A. D., Kellerman, A. M., Battin, T. I., McKenna, A. M., Hood, E., Andino, P., Crespo-Pérez, V., Peter, H., Schön, M., and De Staercke, V.: A tropical cocktail of organic matter sources: Variability in supraglacial and glacier outflow dissolved organic matter composition and age across the Ecuadorian Andes, J. Geophys. Res.-Biogeo., 128, e2022JG007188, https://doi.org/10.1029/2022JG007188, 2023.
Holt, A. D., McKenna, A. M., Kellerman, A. M., Battin, T. I., Fellman, J. B., Hood, E., Peter, H., Schön, M., De Staercke, V., and Styllas, M.: Gradients of deposition and in situ production drive global glacier organic matter composition, Global Biogeochem. Cy., 38, e2024GB008212, https://doi.org/10.1029/2024GB008212, 2024.
Hood, E. and Scott, D.: Riverine organic matter and nutrients in southeast Alaska affected by glacial coverage, Nat. Geosci., 1, 583–587, 2008.
Hood, E., Fellman, J., Spencer, R., Hernes, P., Edwards, R., D'Amore, D., and Scott, D.: Glaciers as a source of ancient and labile organic matter to the marine environment, Letters to Nature, 462, 1044–1048, https://doi.org/10.1038/nature08580, 2009.
Hood, E., Battin, T., Fellman, J., O'Neel, S., and Spencer, R.: Storage and release of organic carbon from glaciers and ice sheets, Nat. Geosci., 8, 91–96, https://doi.org/10.1038/ngeo2331, 2015.
Kohler, T. J., Bourquin, M., Peter, H., Yvon-Durocher, G., Sinsabaugh, R. L., Deluigi, N., Styllas, M., Vanishing Glaciers Field Team, Styllas, M., Schön, M., Tolosano, M., de Staercke, V., and Battin, T. J.: Global emergent responses of stream microbial metabolism to glacier shrinkage, Nat. Geosci., 17, 309–315, 2024.
Kohn, M. J.: Carbon isotope compositions of terrestrial C3 plants as indicators of (paleo) ecology and (paleo) climate, P. Natl. Acad. Sci. USA, 107, 19691–19695, 2010.
McCallister, S. L., Guillemette, F., and Del Giorgio, P. A.: A system to quantitatively recover bacterioplankton respiratory CO2 for isotopic analysis to trace sources and ages of organic matter consumed in freshwaters, Limnol. Oceanogr.-Meth., 4, 406–415, 2006.
McCrimmon, D. O., Bizimis, M., Holland, A., and Ziolkowski, L. A.: Supraglacial microbes use young carbon and not aged cryoconite carbon, Org. Geochem., 118, 63–72, 2018.
McMahon, K. W., Ambrose Jr, W. G., Johnson, B. J., Sun, M.-Y., Lopez, G. R., Clough, L. M., and Carroll, M. L.: Benthic community response to ice algae and phytoplankton in Ny Ålesund, Svalbard, Mar. Ecol. Prog. Ser., 310, 1–14, 2006.
Musilova, M., Tranter, M., Wadham, J., Telling, J., Tedstone, A., and Anesio, A. M.: Microbially driven export of labile organic carbon from the Greenland ice sheet, Nat. Geosci., 10, 360–360, 2017.
Schmidt, S. K., Johnson, B. W., Solon, A. J., Sommers, P., Darcy, J. L., Vincent, K., Vimercati, L., Fountain, A. G., and Porazinska, D. L.: Microbial biogeochemistry and phosphorus limitation in cryoconite holes on glaciers across the Taylor Valley, McMurdo Dry Valleys, Antarctica, Biogeochemistry, 158, 313–326, 2022.
Smith, H. J., Foster, R. A., McKnight, D. M., Lisle, J. T., Littmann, S., Kuypers, M. M., and Foreman, C. M.: Microbial formation of labile organic carbon in Antarctic glacial environments, Nat. Geosci., 10, 356–359, 2017.
Spencer, R. G., Vermilyea, A., Fellman, J., Raymond, P., Stubbins, A., Scott, D., and Hood, E.: Seasonal variability of organic matter composition in an Alaskan glacier outflow: Insights into glacier carbon sources, Environ. Res. Lett., 9, 055005, https://doi.org/10.1088/1748-9326/9/5/055005, 2014.
Spencer, R. G. M., Stubbins, A., Hernes, P. J., Baker, A., Mopper, K., Aufdenkampe, A. K., Dyda, R. Y., Mwamba, V. L., Mangangu, A. M., and Wabakanghanzi, J. N.: Photochemical degradation of dissolved organic matter and dissolved lignin phenols from the Congo River, J. Geophys. Res.-Biogeo., 114, G03010, https://doi.org/10.1029/2009JG000968, 2009.
Stubbins, A., Hood, E., Raymond, P. A., Aiken, G. R., Sleighter, R. L., Hernes, P. J., Butman, D., Hatcher, P. G., Striegl, R. G., and Schuster, P.: Anthropogenic aerosols as a source of ancient dissolved organic matter in glaciers, Nat. Geosci., 5, 198–201, https://doi.org/10.1038/ngeo1403, 2012.
Wang, P., Zhou, W., Xiong, X., Wu, S., Niu, Z., Cheng, P., Du, H., and Hou, Y.: Stable carbon isotopic characteristics of fossil fuels in China, Sci. Total Environ., 805, 150240, https://doi.org/10.1016/j.scitotenv.2021.150240, 2022.
Wilson, F. H., Hults, C. P., Mull, C. G., and Karl, S. M.: Geologic map of Alaska, Scientific Investigations Map 3340, https://doi.org/10.3133/sim3340, 2015.
Xu, L., Roberts, M. L., Elder, K. L., Kurz, M. D., McNichol, A. P., Reddy, C. M., Ward, C. P., and Hanke, U. M.: Radiocarbon in Dissolved Organic Carbon by UV Oxidation: Procedures and Blank Characterization at NOSAMS, Radiocarbon, 63, 357–374, 2021.